Synthesis and biological evaluation of oxygen-, nitrogen-, and sulphur-containing non-fused heterocycles and tricyclic fused pyrimidines
DOI:
https://doi.org/10.14295/bjs.v4i11.810Keywords:
furo-imidazo pyrimidines, thiazolo-thieno pyrimidines, biological activity, bioactivityAbstract
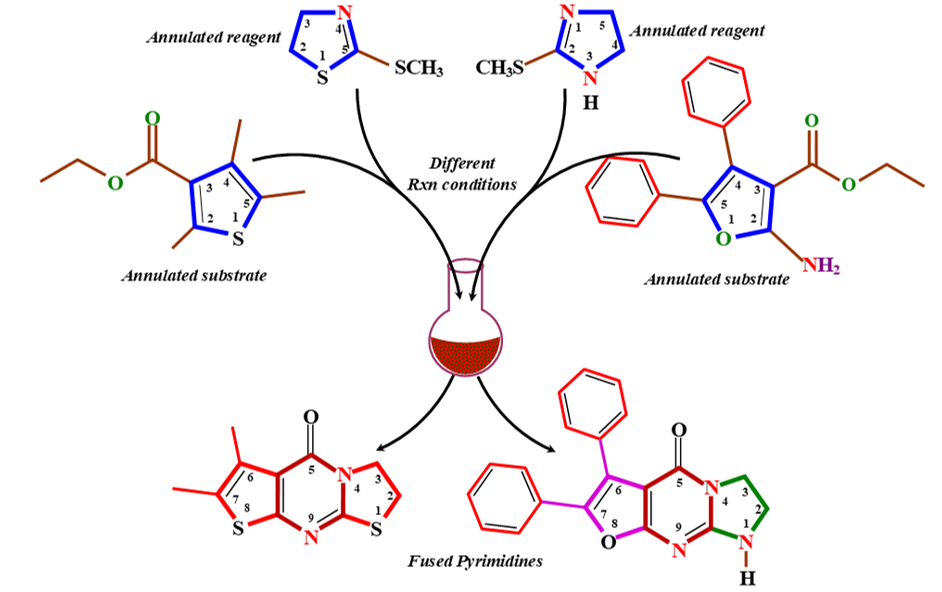
A series of non-fused heterocycles containing oxygen, nitrogen, and sulfur (compounds 2, 3, 6, 9, 10, and 12), as well as furo-imidazo and thiazolo-thieno fused pyrimidines (compounds 7 and 13), were synthesized in good yields using a catalyst-free, convenient, general, and facile method. The molecular structures of all newly synthesized compounds were confirmed by spectral data and elemental microanalyses. Furthermore, the biological activities of all synthesized heterocyclic derivatives were evaluated in vitro against various common pathogenic microorganisms.
References
Azeredo, L. F. S. P., Coutinho J. P., Jabor V. A. P., Feliciano P. R., Nonato M. C., Kaiser C. R., Menezes C. M. S., Hammes, A. S. O., Caffarena, E. R., Hoelz, L. V. B., de Souza, N. B., Pereira, G. A. N., Ceravolo, I. P., Krettli, A. U., & Boechat, N. (2017). Evaluation of 7-arylaminopyrazolo[1,5-a]pyrimidines as anti-Plasmodium falciparum, antimalarial and Pf-dihydroorotate dehydrogenase inhibitors. European Journal of Medicinal Chemistry, 126, 72-83. https://doi.org/10.1016/j.ejmech.2016.09.073 DOI: https://doi.org/10.1016/j.ejmech.2016.09.073
Barot, K. P., Jain, S. V., Gupta, N., Kremer, L., & Singh, S. (2014). Design, synthesis and docking studies of some novel (R)-2-(4’-chlorophenyl)-3-(4’-nitrophenyl)-1, 2, 3, 5-tetrahydrobenzo [4, 5] imidazo [1, 2-c] pyrimidin-4-ol derivatives as antitubercular agents. European Journal of Medicinal Chemistry, 83, 245-255. https://doi.org/10.1016/j.ejmech.2014.06.019 DOI: https://doi.org/10.1016/j.ejmech.2014.06.019
Bassyouni F., Tarek, M., Salama, A., Ibrahim, B., El Dine, S. S., Yassin, N., Hassanein, A., Moharam, M., & Abdel-Rehim, M. (2021). Promising antidiabetic and antimicrobial agents based on fused pyrimidine derivatives: molecular modeling and biological evaluation with histopathological effect. Molecules, 26(8), 2370. https://doi.org/10.3390/molecules26082370 DOI: https://doi.org/10.3390/molecules26082370
Brown D. F., & Kothari D. (1975). Comparison of antibiotic discs from different sources. Journal of Clinical Pathology, 28(10), 779-783. https://doi.org/10.1136/jcp.28.10.779 DOI: https://doi.org/10.1136/jcp.28.10.779
EUCAST. (2021). Antimicrobial susceptibility testing: EUCAST disk diffusion method. In: European Committee on Antimicrobial Susceptibility Testing. Available at: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology. Accessed on 3 November 2025.
Gewald, K. (1966). Heterocyclic enamin esters, versatile synthons in heterocylic synthesis. Chemische Berichte, 99, 94-100.
Gewald, K., Schinke, E., & Botter, H. (1966). Gewald reaction: Syntehsis, properties and applications of substituted 2-aminothipphenes. Chemische Berichte, 99, 94-100. DOI: https://doi.org/10.1002/cber.19660990116
Jansen, I. E., & Mathes, R. A. (1955). The reaction of dithiocarbomate. Journal of American Chemical Society, 77(10), 2866-2868. DOI: https://doi.org/10.1021/ja01615a059
Jansen, I. E., & Mathes, R. A. (1955). Synthesis of thiazinethiols. Journal of American Chemical Society, 77(20), 5431-5432. https://doi.org/10.1021/ja01625a076 DOI: https://doi.org/10.1021/ja01625a076
Jang, M-Y., De Jonghe, S., Segers, K., Anne, J., & Herdewijn, P. (2011). Synthesis of novel 5-amino-thiazolo[4,5-d]pyrimidines as E. coli and S. aureus SecA inhibitors. Bioorganic & Medicinal Chemistry, 19(1), 702-714. https://doi.org/10.1016/j.bmc.2010.10.027 DOI: https://doi.org/10.1016/j.bmc.2010.10.027
Katritzky, A. R., & Rees, C. W. (1984), Structural considerations in the interaction of DNA and acridines. Pergamon Press, 173 p.
Lerman L.S. (1961). Structural considerations in the interaction of DNA and acridines. Journal of Molecular Biology, 3(1), 18-30. https://doi.org/10.1016/S0022-2836(61)80004-1 DOI: https://doi.org/10.1016/S0022-2836(61)80004-1
Luzzati, V., Masson, F., & Lerman, L.S. (1961). Interaction of DNA and proflavine: a small-angle x-ray scattering study. Journal of Molecular Biology, 3(5), 634-639. https://doi.org/10.1016/S0022-2836(61)80026-0 DOI: https://doi.org/10.1016/S0022-2836(61)80026-0
Malnuit, V., Slavetinska, L. P., Nauš, P., Džubák, P., Hajdúch, M., Stolaříková, J., Snášel, J., Pichová, I., & Hocek, M. (2015). 2-Substituted 6-(het)aryl-7-deazapurine ribonucleosides: Synthesis, inhibition of adenosine kinases and antimycobacterial activity. ChemMedChem, 10(6), 1079-1093. https://doi.org/10.1002/cmdc.201500081 DOI: https://doi.org/10.1002/cmdc.201500081
Pogorelčnik, B., Brvar, M., Žegura, B., Filipič, M., Solmajer, T., & Perdih, A. (2015). Discovery of mono- and disubstituted 1H-Pyrazolo[3,4]pyrimidines and 9H-purines as catalytic inhibitors of human DNA Topoisomerase IIα. ChemMedChem, 10(2), 345-359. https://doi.org/10.1002/cmdc.201402459 DOI: https://doi.org/10.1002/cmdc.201402459
Quiroga, J., Romo, P. E., Ortiz, A., Isaza, J. H., Insuasty, B., Abonia, R., Nogueras, M., & Cobo, J. (2016). Synthesis, structures, electrochemical studies and antioxidant activity of 5-aryl-4-oxo-3,4,5,8-tetrahydropyrido[2,3-d]pyrimidine-7-carboxylic acids. Journal of Biomolecular Structure, 1120, 294-301. https://doi.org/10.1016/j.molstruc.2016.05.045 DOI: https://doi.org/10.1016/j.molstruc.2016.05.045
Rashad, A. E., Hegab, M. I., Abdel-Megeid, R. E., Micky, J. A., & Abdel-Megeid, F. M. E. (2008). Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorganic & Medicinal Chemistry, 16(15), 7102-7106. https://doi.org/10.1016/j.bmc.2008.06.054 DOI: https://doi.org/10.1016/j.bmc.2008.06.054
Tozkoparan, B., Ertan, M., Kelicen, P., & Demirdamar, R. (1999). Synthesis and anti-inflammatory activities of some thiazolo[3,2-a]pyrimidine derivatives. Il Farmaco, 54(9), 588-593. https://doi.org/10.1016/S0014-827X(99)00068-3 DOI: https://doi.org/10.1016/S0014-827X(99)00068-3
Vijaya Raj, K. K., Narayana, B., & Suchetha Kumari, N. (2006). New thiazoles containing pyrazolopyrimidine moiety as possible analgesic agents. Journal of Pharmacology and Toxicology, 1(6), 559-565. https://doi.org/10.3923/jpt.2006.559.565 DOI: https://doi.org/10.3923/jpt.2006.559.565
Wang, Y., Mitchell-Ryan, S., Raghavan, S., George, C., Orr, S., Hou, Z., Matherly, L. H., Gangjee, A. (2015). Novel 5-substituted pyrrolo[2,3-d]pyrimidines as dual inhibitors of glycinamide ribonucleotide formyltransferase and 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase and as potential antitumor agents. Journal of Medicinal Chemistry, 58(3), 1479-1493. https://doi.org/10.1021/jm501787c DOI: https://doi.org/10.1021/jm501787c
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Mohammad Mizanur Rahman, Rabiul Islam, Masud Reza, Fakir Rafiqul Alam

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


