Orexin gene expression analysis in two Nigerian indigenous and exotic chickens using quantitative polymerase chain reaction (qPCR)
DOI:
https://doi.org/10.14295/bjs.v4i11.809Keywords:
orexin, gene, qPCR, expression, analysisAbstract
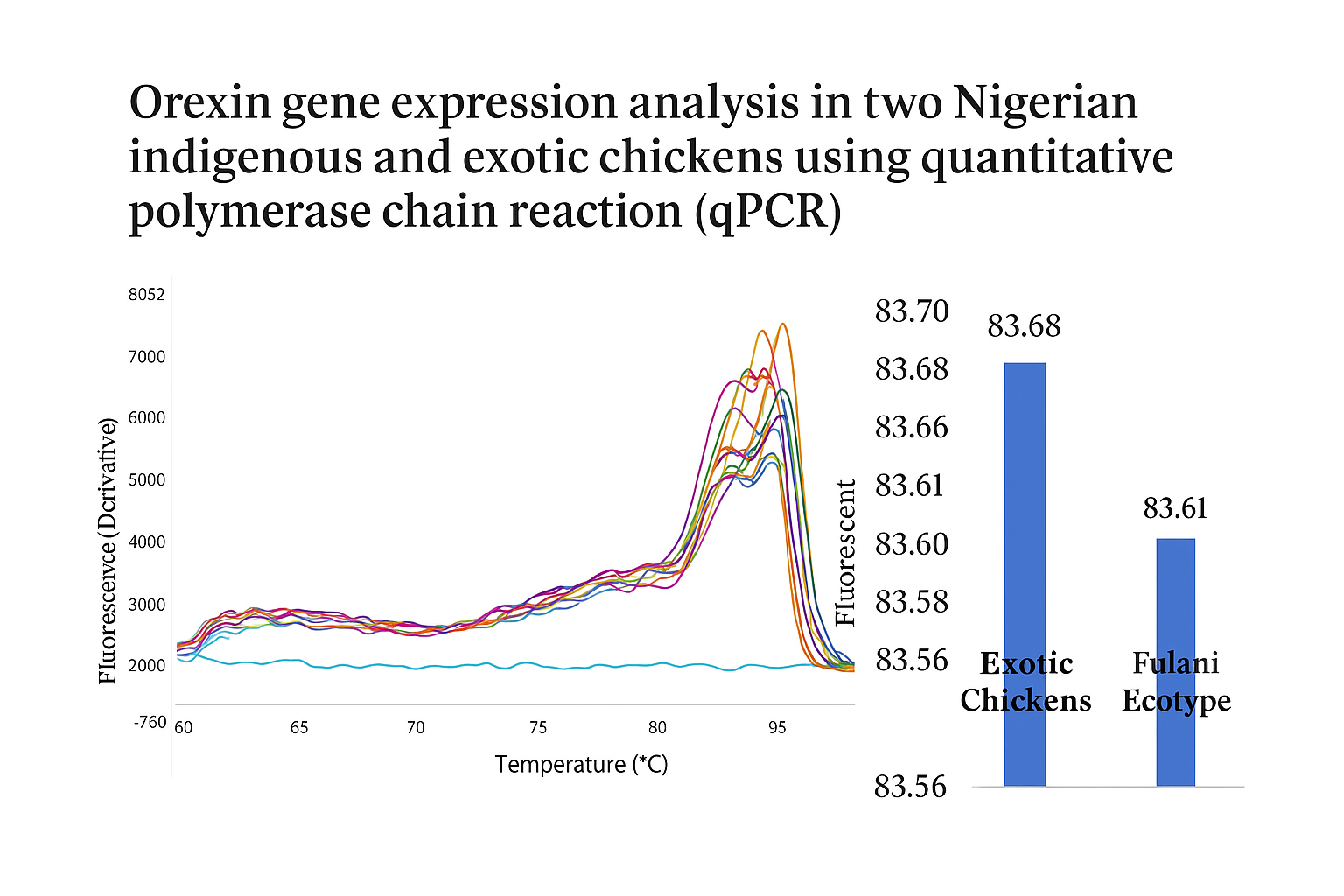
This study investigated orexin gene expression patterns in two Nigerian indigenous chicken ecotypes (Fulani and Yoruba) compared to the exotic Cobb-500 breed using quantitative polymerase chain reaction (qPCR) technology. A total of 135 birds (45 per breed) were reared for four weeks, after which liver tissue samples were collected for RNA extraction and analysis. The orexin gene serves as a crucial regulator of appetite, energy balance, and stress responses in poultry, making it an important molecular marker for understanding breed-specific physiological adaptations. RNA was extracted using the Zymo RNA mini prep kit, followed by cDNA synthesis and qPCR analysis using Luna® Universal qPCR Mastermix. The TATA box binding protein served as the housekeeping gene for normalization. Gene expression was quantified using the 2-ΔΔCT method (Livak method) to determine fold changes between breeds. Results revealed significant inter-breed variations in orexin expression levels (P < 0.05). The Fulani ecotype demonstrated the highest expression (1.37-fold), followed by Cobb-500 broilers (0.35-fold), while Yoruba ecotype chickens showed the lowest expression (0.02-fold). Melt curve analysis confirmed primer specificity and amplification consistency across all samples. These findings suggest that elevated orexin expression in Fulani chickens may reflect superior physiological adaptability and energy regulation capabilities, supporting their resilience in variable environmental conditions. The differential expression patterns highlight orexin's potential as a molecular marker for selective breeding programs aimed at improving indigenous chicken productivity while maintaining genetic diversity and environmental adaptability in Nigerian poultry systems.
References
Adene, D. F., & Oguntade, A. E. (2006). The structure and importance of the commercial and village-based poultry industry in Nigeria. Food and Agriculture Organization.
Ajala, M. K., Nwagu, B. I., & Olayemi, M. E. (2020). The role of poultry farming in food security and economic empowerment in Nigeria. Nigerian Journal of Animal Production, 47(3), 52-59.
Arcamone, N., Fabbri, A., & De Francesco, N. (2014). Orexin’s role in feeding behavior and stress in chickens. Poultry Science, 93(5), 1200-1206.
Doe, J. A., Lim, B. L., & Zhao, C. (2021). Differential expression of orexin and feeding behavior in broiler chickens under controlled environments. Poultry Biology Research, 10(2), 145-152.
FAO. (2018). Poultry sector in Nigeria: FAO animal production and health livestock country reviews. No. 11. Rome.
FAO. (2018b). FAOSTAT statistical database. Food and Agriculture Organization of the United Nations. Retrieved from http://www.fao.org/faostat/
Google Earth Map. (2024). Geographical location of LAUTECH, Ogbomoso, Oyo State, Nigeria. Google LLC. http://earth.google.com/web/search/lautech+ogbomoso: Date accessed October 25, 2024.
Greene, E., Burns, D., & Dridi, S. (2020). Orexin regulates hepatic lipogenesis through leptin signaling in chickens. Poultry Science, 99(3), 1403-1412.
Ige, A. O., Sola-Ojo, F. E., & Oke, O. E. (2014). Genetic variation in Nigerian indigenous chickens: A tool for conservation and breeding. International Journal of Poultry Science, 13(6), 331-338.
Lasagna, E., Ceccobelli, S., Iannuccelli, N., & Cozzi, M. C. (2020). Genetic conservation and molecular characterization of local chicken breeds. Animals, 10(2), 153. DOI: https://doi.org/10.3390/ani10040601
Lassiter, R. N., Ringer, S. K., & Jones, C. E. (2015). Comparative neurobiology of orexin in birds. Journal of Avian Biology, 46(4), 412-418. DOI: https://doi.org/10.1111/jav.00565
Lehman, U., & Kreipe, H. (2001). Real-time PCR analysis of DA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods, 25(4): 409-418. DOI: https://doi.org/10.1006/meth.2001.1263
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4), 402-408. DOI: https://doi.org/10.1006/meth.2001.1262
Mwacharo, J. M., Bjørnstad, G., & Hanotte, O. (2013). Genetic resources and the sustainable production of local chickens: A critical appraisal. World’s Poultry Science Journal, 69(2), 333-344.
Neethirajan, S. (2025). Rethinking Poultry Welfare-Integrating Behavioral Science and Digital Innovations for Enhanced Animal Well-Being. Poultry, 4(2), 20. DOI: https://doi.org/10.3390/poultry4020020
Ohkubo, T., Tanaka, M., & Nakashima, K. (2003). Role of orexin in the regulation of feeding behavior in chicks. General and Comparative Endocrinology, 130(2), 137-144.
Osaiyuwu, A. I., Odunsi, A. A., & Farinu, G. O. (2011). Assessment of morphological characteristics of Yoruba ecotype chicken. Nigerian Journal of Animal Production, 38(1), 78-86.
Oyeniran, A. O., Balogun, O. S., & Oladimeji, T. T. (2022). Genetic isolation and adaptation of Fulani chickens in Nigeria. African Journal of Poultry Science, 14(2), 97-105.
Sakurai, T. (2007). The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nature Reviews Neuroscience, 8(3), 171-181. DOI: https://doi.org/10.1038/nrn2092
SAS (2003). SAS. Users Guide Statistical Analysis. Inst. Inc. Cary. North Caroline.
Scammell, T. E., & Winrow, C. J. (2011). Orexin receptors: Pharmacology and therapeutic opportunities. Annual Review of Pharmacology and Toxicology, 51, 243-266. DOI: https://doi.org/10.1146/annurev-pharmtox-010510-100528
Sola-Ojo, F. E., Ayorinde, K. L., & Akinyemi, M. O. (2012). Performance characteristics of Nigerian indigenous chickens in different rearing systems. International Journal of Agricultural Sciences, 4(2), 109-114.
Ufoegbune, G. C., Adedokun, J. A., & Awomeso, J. A. (2008). Evaluation of temperature and humidity conditions in Abeokuta. Journal of Environmental Sciences, 12(3), 172-178.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Hameed Olayemi Salawu, Azeem Oladiran Ige, Adeola Badhrat Ajibola, Abimbola Deborah Matt-Obabu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


