In silico evaluation of twenty-five amino derivatives as potential nitric oxide synthase inhibitors
DOI:
https://doi.org/10.14295/bjs.v4i8.751Keywords:
amino derivatives, nitric oxide synthase, cancerAbstract
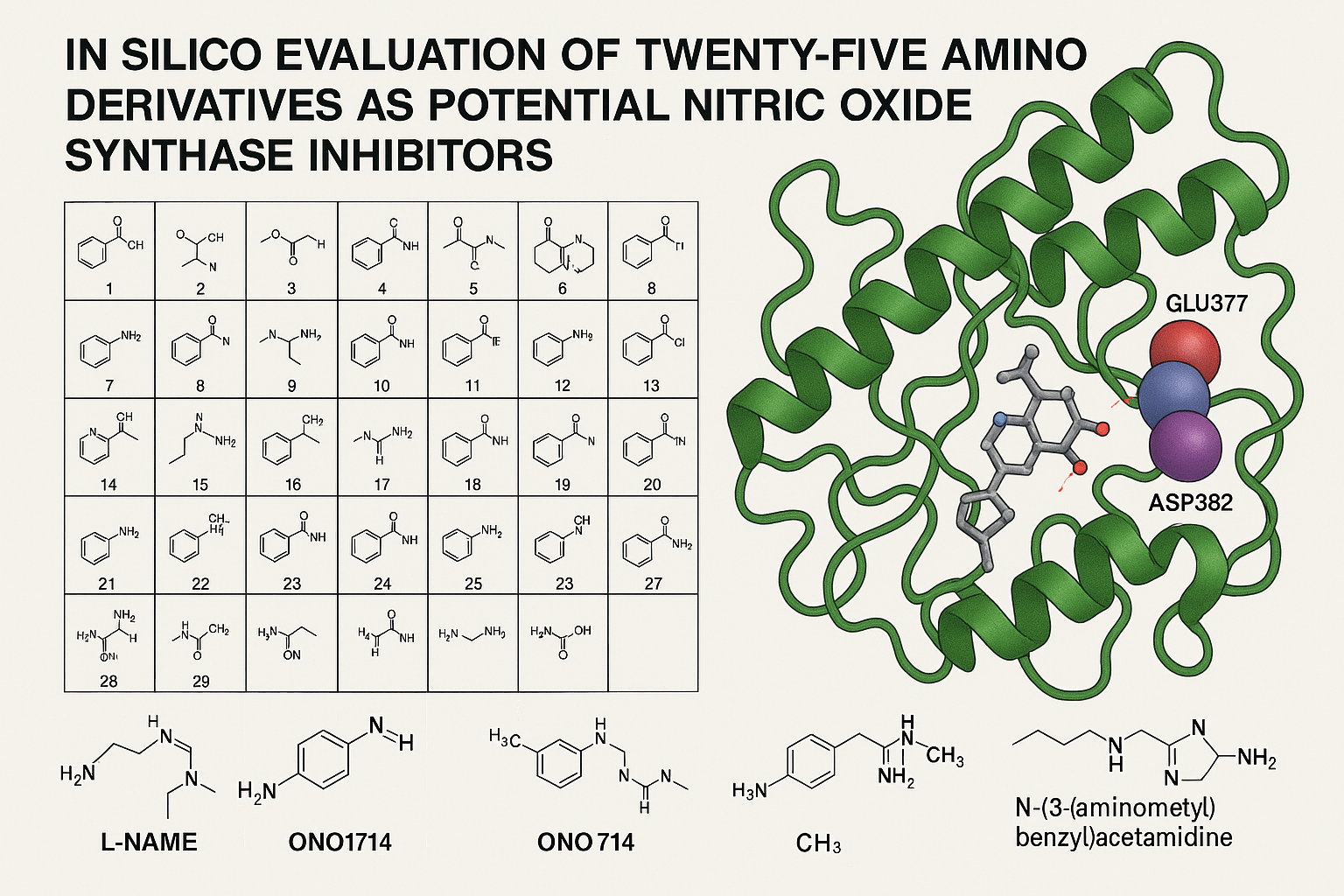
There are studies indicating that nitric oxide synthase can be involved in cancer cell growth. It is important to mention that some inhibitors of nitric oxide synthase can produce changes in cancer cell growth. However, there is little information on the interaction of some amino derivatives with nitric oxide synthase surface. The aim of this research was to determine the theoretical interaction of amino derivatives (compounds 1-25) with nitric oxide synthase using the 4d1o protein as a tool. Besides, L-NAME, ONO1714, and N-(3-(aminomethyl)benzyl)acetamidine drugs were used as controls in the DockingServer program. The results showed differences in the number of aminoacid residues and energy levels involved in the interaction of amino derivatives with the 4d1o protein surface compared with the controls. Furthermore, the inhibition constants for amino derivatives 4, 15, 20, 24, and 25 were lower compared to L-NAME and ONO1714 drugs. In conclusion, these theoretical results indicate that compounds 4, 15, 20, 24, and 25 have a higher affinity for the 4d1o protein surface. This data indicates that amino derivatives 4, 15, 20, 24, and 25 can exert changes in the biological activity of nitric oxide synthase. This phenomenon could translate into a decrease in cancer cell growth; however, to validate this hypothesis, it is necessary to perform different experiments in a biological model.
References
Aminian, A., Wilson, R., Al-Kurd, A., Tu, C., Milinovich, A., & Kroh, M. (2022). Association of bariatric surgery with cancer risk and mortality in adults with obesity. Journal of American Medical Asocciation, 327(24), 2423-33. https://doi.org/10.1001/jama.2022.9009 DOI: https://doi.org/10.1001/jama.2022.9009
Askerova, U. F. (2023). Prediction of acute toxicity for (Z)-3-(2-phenylhydrazinylidene) benzofuran-2 (3H)-one and its derivatives for rats using GUSAR program. New Materials, Compounds and Applications, 7(1), 50-56.
Bakchi, B., Krishna, A. D., Sreecharan, E., Ganesh, V. B. J., Niharika, M., Maharshi, S., & Shaik, A. B. (2022). An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: a medicinal chemist's perspective. Journal of Molecular Structure, 1259, 132712. https://doi.org/10.1016/j.molstruc.2022.132712 DOI: https://doi.org/10.1016/j.molstruc.2022.132712
Banerjee, P., Eckert, A. O., Schrey, A. K., & Preissner, R. (2018). ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic acids research, 46(W1), W257-W263. https://doi.org/10.1093/nar/gky318 DOI: https://doi.org/10.1093/nar/gky318
Bikadi, Z., Hazai, E. (2009). Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. Journal of Cheminformatics, 1, 15. DOI: https://doi.org/10.1186/1758-2946-1-15
Bugnon, M., Röhrig, U. F., Goullieux, M., Perez, M. A., Daina, A., Michielin, O., & Zoete, V. (2024). SwissDock 2024: major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic acids research, 52(W1), W324-W332. https://doi.org/10.1093/nar/gkae300 DOI: https://doi.org/10.1093/nar/gkae300
Cahlin, C., Gelin, J., Delbro, D., Lönnroth, C., Doi, C., & Lundholm, K. (2000). Effect of cyclooxygenase and nitric oxide synthase inhibitors on tumor growth in mouse tumor models with and without cancer cachexia related to prostanoids. Cancer Research, 60(6), 1742-1749.
Chiarelli, L. R., Mori, M., Barlocco, D., Beretta, G., Gelain, A., Pini, E., & Meneghetti, F. (2018). Discovery and development of novel salicylate synthase (MbtI) furanic inhibitors as antitubercular agents. European Journal of Medicinal Chemistry, 155, 754-763. https://doi.org/10.1016/j.ejmech.2018.06.033 DOI: https://doi.org/10.1016/j.ejmech.2018.06.033
Figueroa-Valverde, L., Rosas-Nexticapa, M., Alvarez-Ramirez, M., Aguilar-Sanchez, E., Mateu-Armad, M. V., & Bonilla-Zavaleta, E. (2024). Interaction of some chalcone derivatives with calcium channels using a theoretical model. Brazilian Journal of Science, 3(11), 1-15. https://doi.org/10.14295/bjs.v3i11.658 DOI: https://doi.org/10.14295/bjs.v3i11.658
Fujimoto, H., Ando, Y., Yamashita, T., Terazaki, H., Tanaka, Y., Sasaki, J., & Ando, M. (1997). Nitric oxide synthase activity in human lung cancer. Japanese Journal of Cancer Research, 88(12), 1190-1198. https://doi.org/10.1111/j.1349-7006.1997.tb00348.x DOI: https://doi.org/10.1111/j.1349-7006.1997.tb00348.x
Gao, Y., Zhou, S., Xu, Y., Sheng, S., Qian, S. Y., & Huo, X. (2019). Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide, 83, 33-39. https://doi.org/10.1016/j.niox.2018.12.008 DOI: https://doi.org/10.1016/j.niox.2018.12.008
Halgren. (1998). Merck molecular force field. I. Basis, form, scope, parametrization, and performance of MMFF94. Journal of Computational Chemistry, 17(5-6), 490-519. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P
Hazai, E., Kovács, S., Demkó, L., & Bikádi, Z. (2009). DockingServer: molecular docking calculations online. Acta pharmaceutica Hungarica, 79(1), 17-21.
Hecht, S., & Hatsukami, D. (2022). Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nature Reviews Cancer, 22(3), 143-155. DOI: https://doi.org/10.1038/s41568-021-00423-4
Huang, F., & Yu, S. (2018). Esophageal cancer: risk factors, genetic association, and treatment. Asian Journal Surgery, 41(3), 210-215. https://doi.org/10.1016/j.asjsur.2016.10.005 DOI: https://doi.org/10.1016/j.asjsur.2016.10.005
Im, P., Yang, L., Kartsonaki, C., Chen, Y., Guo, Y., & Du, H. (2022). Alcohol metabolism genes and risks of site‐specific cancers in Chinese adults: An 11‐year prospective study. International Journal of Cancer 150(10),1627-39. https://doi.org/10.1002/ijc.33917 DOI: https://doi.org/10.1002/ijc.33917
Kampa, M., Hatzoglou, A., Notas, G., Niniraki, M., Kouroumalis, E., & Castanas, E. (2001). Opioids are non-competitive inhibitors of nitric oxide synthase in T47D human breast cancer cells. Cell Death & Differentiation, 8(9), 943-952. DOI: https://doi.org/10.1038/sj.cdd.4400893
Khrapova, M. V., Khrapov, S. E., Chechushkov, A. V., Kozhin, P. M., Romakh, L. P., Serykh, A. E., & Menshchikova, E. B. (2023). The toxicity of a new monophenolic synthetic inducer of Keap1/Nrf2/ARE redox-sensitive signaling system in vitro and in vivo. Cell and Tissue Biology, 17(3), 299-305. DOI: https://doi.org/10.1134/S1990519X23030069
Kong, R., Yang, G., Xue, R., Liu, M., Wang, F., Hu, J., & Chang, S. (2020). COVID-19 Docking Server: a meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics, 36(20), 5109-5111. https://doi.org/10.1093/bioinformatics/btaa645 DOI: https://doi.org/10.1093/bioinformatics/btaa645
Krishnamoorthy, P. K., Balaraman, A. D., Priyadharshini, A., Shanmugam, D. A., Muthukumaran, S., Kesavamurthy, A., & Revanasiddappa, P. D. (2023). Molecular docking and simulation binding analysis of boeravinone B with caspase-3 and EGFR of hepatocellular carcinoma. Letters in Drug Design & Discovery, 20(2), 238-244. https://doi.org/10.2174/1570180819666220805163725 DOI: https://doi.org/10.2174/1570180819666220805163725
Lazarus, E., & Bays, H. (2022). Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS). Obesity Pill, 3,100026. https://doi.org/10.1016/j.obpill.2022.100026
Lazarus, E., & Bays, H. (2022). Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillarys, 3, 100026. https://doi.org/10.1016/j.obpill.2022.100026 DOI: https://doi.org/10.1016/j.obpill.2022.100026
Lee, A., Yang, X., Tyrer, J., Gentry-Maharaj, A., Ryan, A., Mavadda, N., & Antoniou, A. Comprehensive epithelial tubo-ovarian cancer risk prediction model incorporating genetic and epidemiological risk factors. Journal of Medical Genetics, 2022; 59(7), 632-643. DOI: https://doi.org/10.1136/jmedgenet-2021-107904
Liu, C. Y., Wang, C. H., Chen, T. C., Lin, H. C., Yu, C. T., & Kuo, H. P. (1998). Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. British Journal of Cancer, 78(4), 534-541. DOI: https://doi.org/10.1038/bjc.1998.528
Lohachova, K. O., Sviatenko, A. S., Kyrychenko, A., Ivanov, V. V., Langer, T., Kovalenko, S. M., & Kalugin, O. N. (2024). Computer-aided drug design of novel nirmatrelvir analogs inhibiting main protease of Coronavirus SARS-CoV-2. Journal of Applied Pharmaceutical Science, 14(5), 232-239. https://dx.doi.org/10.7324/JAPS.2024.158114 DOI: https://doi.org/10.7324/JAPS.2024.158114
Loibl, S., Buck, A., Strank, C., von Minckwitz, G., Roller, M., Sinn, H. P., & Kaufmann, M. (2005). The role of early expression of inducible nitric oxide synthase in human breast cancer. European Journal of Cancer, 41(2), 265-271. https://doi.org/10.1016/j.ejca.2004.07.010 DOI: https://doi.org/10.1016/j.ejca.2004.07.010
Loibl, S., Von Minckwitz, G., Weber, S., Sinn, H. P., Schini‐Kerth, V. B., Lobysheva, I., & Kaufmann, M. (2002). Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer, 95(6), 1191-1198. https://doi.org/10.1002/cncr.10817 DOI: https://doi.org/10.1002/cncr.10817
Minhas, R., Bansal, Y., & Bansal, G. (2020). Inducible nitric oxide synthase inhibitors: A comprehensive update. Medicinal Research Reviews, 40(3), 823-855. https://doi.org/10.1002/med.21636 DOI: https://doi.org/10.1002/med.21636
Morris, M., Goodsell, D., Hallyday, R., Huey, R., hart, W., Belew, R., & Olson, A. (1998). Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of Computational Chemistry, 19(14), 1639-1662. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14%3C1639:AID-JCC 10%3E3.0.CO;2-B DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
O’Sullivan, D., Sutherland, R., Town, S., Chow, K., Fan, J., Forbes, N., & Brenner, D. (2022). Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology, 20(6), 1229-1240. https://doi.org/10.1016/j.cgh.2021.01.037 DOI: https://doi.org/10.1016/j.cgh.2021.01.037
Phua, Z., MacInnis, R., % Jayasekara, H. (2022). Cigarette smoking and risk of second primary cancer: a systematic review and meta-analysis. Cancer Epidemiology, 78, 102160. https://doi.org/10.1016/j.canep.2022.102160 DOI: https://doi.org/10.1016/j.canep.2022.102160
Qing, X., Yin Lee, X., De Raeymaeker, J., RH Tame, J., YJ Zhang, K., De Maeyer, M., & RD Voet, A. (2014). Pharmacophore modeling: advances, limitations, and current utility in drug discovery. Journal of Receptor, Ligand and Channel Research, 81-92. DOI: https://doi.org/10.2147/JRLCR.S46843
Saad, M., Mokrab, Y., Halabi, N., Shan, J, Razali R, Kunji K, & Chouchane L. Genetic predisposition to cancer across people of different ancestries in Qatar: A population-based, cohort study. The Lancet Oncology, 2022; 23(3), 341-352. DOI: https://doi.org/10.1016/S1470-2045(21)00752-X
Schaller, D., Šribar, D., Noonan, T., Deng, L., Nguyen, T. N., Pach, S., & Wolber, G. (2020). Next generation 3D pharmacophore modeling. Wiley Interdisciplinary Reviews: Computational Molecular Science, 10(4), e1468. https://doi.org/10.1002/wcms.1468 DOI: https://doi.org/10.1002/wcms.1468
Siegel, R., Miller, K., Fuchs, H., Jemal, A. (2022). Cancer statistics. CA Cancer Journal for Clinicians, 72(1), 7-33. https://doi.org/10.3322/caac.21708 DOI: https://doi.org/10.3322/caac.21708
Solis, F., & Wets, R. (1981). Minimization by Random Search Techniques. Mathematics of Operations Research, 6 (1), 19-30. https://doi.org/10.1287/moor.6.1.19 DOI: https://doi.org/10.1287/moor.6.1.19
Sushko, I., Salmina, E., Potemkin, V. A., Poda, G., & Tetko, I. V. (2012). ToxAlerts: a web server of structural alerts for toxic chemicals and compounds with potential adverse reactions. Journal of Chemical Information and Modeling, 52(8), 2310-2316. https://doi.org/10.1021/ci300245q DOI: https://doi.org/10.1021/ci300245q
Swan, J., Szabó, Z., Peters, J., Kummu, O., Kemppi, A., Rahtu-Korpela, L, Magga, J. (2024). Inhibition of activin receptor 2 signalling ameliorates metabolic dysfunction–associated steatotic liver disease in western diet/L-NAME induced cardiometabolic disease. Biomedicine & Pharmacotherapy, 175, 116683. https://doi.org/10.1016/j.biopha.2024.116683 DOI: https://doi.org/10.1016/j.biopha.2024.116683
Tu, K., Ma, T., Zhou, R., Xu, L., Fang, Y., & Zhang, C. (2022). Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China. Nutrients, 14(20), 4375. https://doi.org/10.3390/nu14204375 DOI: https://doi.org/10.3390/nu14204375
Verdonk, M. L., Cole, J. C., Hartshorn, M. J., Murray, C. W., & Taylor, R. D. (2003). Improved protein–ligand docking using GOLD. Proteins: Structure, Function, and Bioinformatics, 52(4), 609-623. https://doi.org/10.1002/prot.10465 DOI: https://doi.org/10.1002/prot.10465
Wan, Y., Wu, K., Wang, L., Yin, K., & Song, M. (2022). Dietary fat and fatty acids in relation to risk of colorectal cancer. European Journal of Nutrition, 61(4), 1863-1873. DOI: https://doi.org/10.1007/s00394-021-02777-9
Wolber, G., & Kosara, R. (2006). Pharmacophores from macromolecular complexes with LigandScout. Pharmacophores and pharmacophore searches, 32, 131-150. https://doi.org/ 10.1002/3527609164 DOI: https://doi.org/10.1002/3527609164.ch6
Wolber, G., & Langer, T. (2005). LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. Journal of chemical information and modeling, 45(1), 160-169. https://doi.org/10.1021/ci049885e DOI: https://doi.org/10.1021/ci049885e
Xia, C., Dong, X., Li, H., Cao, M., Sun, D., He, S., & Chen, W. (2022). Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chinese Medical Journal, 135(5), 584-590. DOI: https://doi.org/10.1097/CM9.0000000000002108
Yagihashi, N., Kasajima, H., Sugai, S., Matsumoto, K., Ebina, Y., Morita, T., & Yagihashi, S. (2000). Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virchows Archiv, 436, 109-114. DOI: https://doi.org/10.1007/PL00008208
Yang, S. Y. (2010). Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Discovery Today, 15(11-12), 444-450. https://doi.org/10.1016/j.drudis.2010.03.013 DOI: https://doi.org/10.1016/j.drudis.2010.03.013
Yoo, J., Han, K., Shin, D., Kim, D., Kim, B., & Chun, S. (2022). Association Between Changes in Alcohol Consumption and Cancer Risk. Journal of American Medical Association, 5(8), e2228544. https://doi.org/10.1001/jamanetworkopen.2022.28544 DOI: https://doi.org/10.1001/jamanetworkopen.2022.28544
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Regina Cauich-Carrillo, Marcela Rosas Nexticapa, Magdalena Alvarez-Ramirez, Maria Virginia Mateu-Armad, Emilio Aguilar-Sanchez, Lauro Figueroa-Valverde

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


