Effect of different osteogenic media and saline solutions on the osteogenesis protocol using MC3T3-E1 subclones
DOI:
https://doi.org/10.14295/bjs.v4i1.662Keywords:
osteoblast, cell line, alizarin red, alkaline phosphatase, saline solutionsAbstract
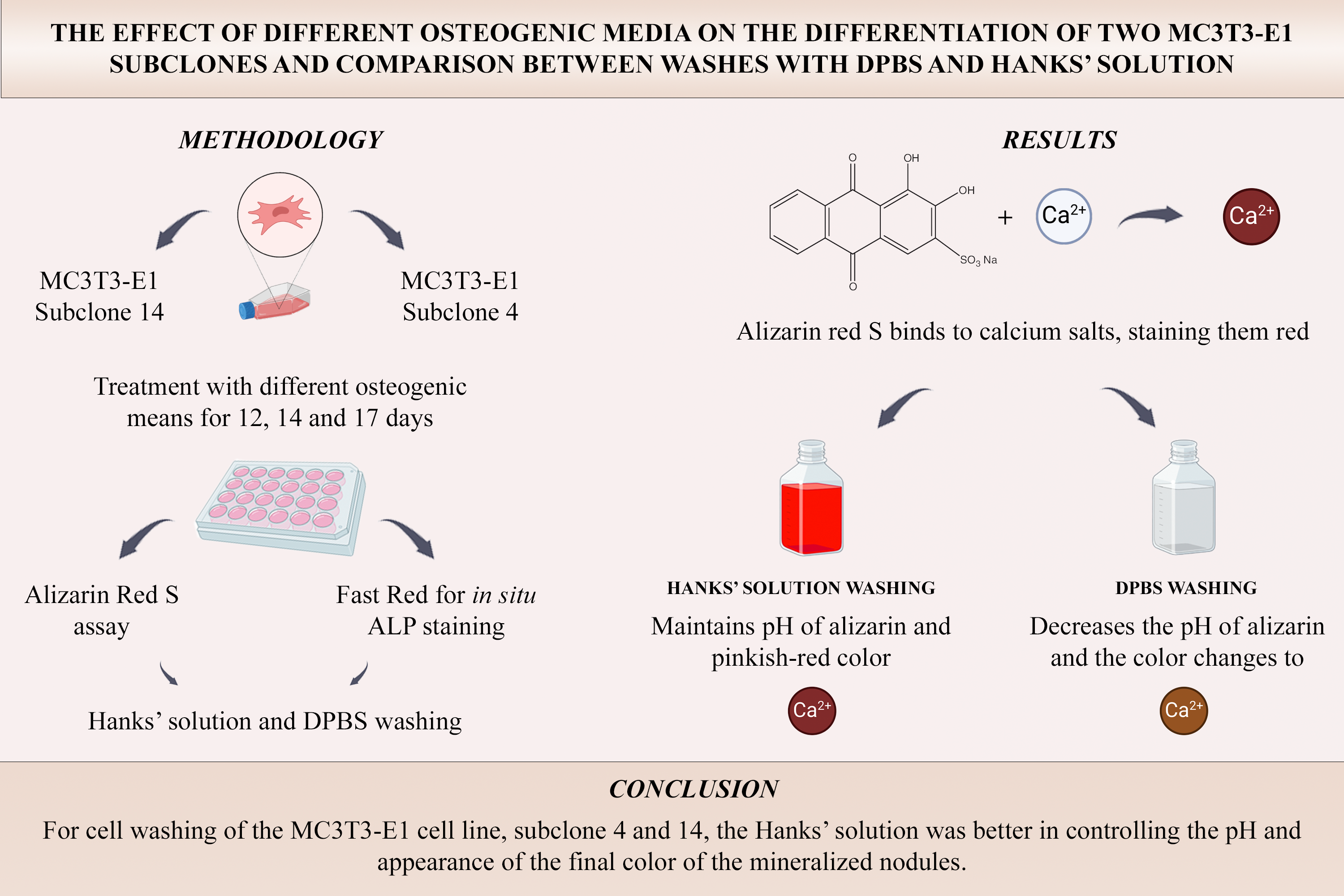
Adequate laboratory protocols may improve the study of bone tissue and its metabolism. Thus, the use of effective techniques for staining bone cells in vitroand evaluating their function is significant. The cell line used for this purpose was MC3T3-E1, which contains preosteoblasts with mineralization potential. Several osteogenic media are used in the culture of these cells, and a variety of saline solutions are used for washing cultures in mineralization staining protocols and in situ alkaline phosphatase detection. Thus, the objective of the present study was to evaluate the effects of different osteogenic media in the culture of MC3T3-E1 cells, subclones 4 and 14, in addition to washing with Dulbecco’s phosphate saline solution (DPBS) and Hanks’ balanced salt solution (HBSS) in an alizarin red staining assay and situ alkaline phosphatase labeling via the Fast Red method. The cells were seeded at a density of 1x104 cells/well for 7 and 10 days for the Fast Red assay and 12, 14, and 17 days for the staining of mineralization nodules. The data were statistically analyzed and significance was set for p < 0.05. Data obtained showed that the presence of dexamethasone significantly enhanced ALP detection in subclone 14 osteoblastic cells after 7 and 10 days as well as in subclone 4 cells washed with DPBS after 7 and 10 days when compared to control. Washing with Hanks’ solution significantly increased the quantification of ALP at 10 days and of mineralized nodules in 4 subclone cells after 17 days. Moreover, alizarin red staining improved, resulting in a more intense red color, in the group that was washed with Hanks’ solution for both subclones in all experimental periods. Thus, it is suggested that washing with Hanks’ salt solution is better for in vitro staining of calcium nodules when using the alizarin red method.
References
Alves, E. A., & Guimarães, A. C. R. (2010). Cultivo celular. In: Molinaro, Etelcia Moraes; Caputo, Luzia Fátima Gonçalves; Amendoeira, Maria Regina Reis (Org.). Conceitos e métodos para a formação de profissionais em laboratórios de saúde (2010). V.2. Rio de Janeiro: EPSJV, p. 215-253.
Brasil. (2024). Balanced salt solutions, Thermo Fisher Scientific Inc. A. Available in: <https://www.thermofisher.com/br/en/home/life-science/cell-culture/mammalian-cell-culture/reagents/balanced-salt-solutions.html>. Access on: 29 de maio de 2024.
Carrel, A. (1912). On the permanent life of tissues outside of the organism. The Journal of Experimental Medicine, 15(5), 516-528. https://doi.org/10.1084/jem.15.5.516 DOI: https://doi.org/10.1084/jem.15.5.516
Cheng, S. L., Zhang, S. F., & Avioli, L. V. (1996). Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. Journal of Cellular Biochemistry, 61(2), 182-193. https://doi.org/10.1002/(SICI)1097-4644(19960501)61:2%3C182::AID-JCB3%3E3.0.CO;2-Q DOI: https://doi.org/10.1002/(SICI)1097-4644(19960501)61:2<182::AID-JCB3>3.0.CO;2-Q
Freshney, R. I. (1994). Culture of animal cells: A manual of basic technique. 4. ed. Nova York: Wiley-Liss, p. 397.
Forsling, W., & Wu, L. (1993). Surface complexation at hydrous fluorapatite. Aquatic Science, 55, 336-346. https://doi.org/10.1007/BF00877278 DOI: https://doi.org/10.1007/BF00877278
Fu, K., Yang, L. L., Gao, N., Liu, P., Xue, B., He, W., Qiu, W., & Wen, X. (2024) Modified five times simulated body fluid for efficient biomimetic mineralization. Heliyon, 10(12), e32850. https://doi.org/10.1016/j.heliyon.2024.e32850. DOI: https://doi.org/10.1016/j.heliyon.2024.e32850
Gregory, C. A., Gunn, W. G., & Peister, A. (2004). An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Analytical Biochemistry, 329(1), 77-84. https://doi.org/10.1016/j.ab.2004.02.002 DOI: https://doi.org/10.1016/j.ab.2004.02.002
Hanks, J. (1976). Hanks’ balanced salt solution and pH control. TCA manual/Tissue Culture Association, 1, 3-4. https://doi.org/10.1007/BF00914425 DOI: https://doi.org/10.1007/BF00914425
Hanks, J. H., & Wallace, R. E. (1949). Relation of oxygen and temperature in the preservation of tissues by refrigeration. Experimental Biology and Medicine, 71(2), 196-200. https://doi.org/10.3181/00379727-71-17131 DOI: https://doi.org/10.3181/00379727-71-17131
Hwang, P. W., & Horton, J. A. (2019) Variable osteogenic performance of MC3T3-E1 subclones impacts their utility as models of osteoblast biology. Scientific Reports, 9(1), 8299. https://doi.org/10.1038/s41598-019-44575-8 DOI: https://doi.org/10.1038/s41598-019-44575-8
Izumiya, M., Haniu, M., Ueda, K., Ishida, H., Ma, C., Ideta, H., Sobajima, A., Ueshiba, K., Uemura, T., Saito, N., & Haniu, H. (2021). Evaluation of MC3T3-E1 cell osteogenesis in different cell culture media. International Journal of Molecular Sciences, 22(14), 7752. https://doi.org/10.3390/ijms22147752. DOI: https://doi.org/10.3390/ijms22147752
Lemlikchi, W., Sharrock, P., Fiallo, M., Nzihou, A., & Mecherri, M. -O. (2014). Hydroxyapatite and alizarin sulfonate ARS modeling interactions for textile dyes removal from wastewaters. Procedia Engineering, 83, 378-385. https://doi.org/10.1016/j.proeng.2014.09.032 DOI: https://doi.org/10.1016/j.proeng.2014.09.032
Li, W., Zhang, S., Liu, J., Liu, Y., & Liang, Q. (2019). Vitamin K2 stimulates MC3T3E1 osteoblast differentiation and mineralization through autophagy induction. Molecular Medicine Reports, 19(5), 3676-3684. https://doi.org/10.3892/mmr.2019.10040 DOI: https://doi.org/10.3892/mmr.2019.10040
Majors, A. K., Boehm, C. A., Nitto, H., Midura, R. J., & Muschler, G. F. (1997). Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. Journal of Orthopaedic Research, 15(4), 546-557. https://doi.org/10.1002/jor.1100150410 DOI: https://doi.org/10.1002/jor.1100150410
Petrakova, N. V., Teterina, A. Y., Mikheeva, P. V., Akhmedova, S. A., Kuvshinova, E. A., Sviridova, I. K., Sergeeva, N. S., Smirnov, I. V., Fedotov, A. Y., Kargin, Y. F, Barinov, S. M., & Komlev, V. S. (2021). In Vitro study of octacalcium phosphate behavior in different model solutions. ACS Omega, 6(11), 7487-7498. https://doi.org/10.1021/acsomega.0c06016 DOI: https://doi.org/10.1021/acsomega.0c06016
Puchtler, H., Meloan, S. N., & Terry, M. S. (1969). On the history and mechanism of alizarin and alizarin red S stains for calcium. Journal of Histochemistry & Cytochemistry, 17(2), 110-124. https://doi.org/10.1177/17.2.110 DOI: https://doi.org/10.1177/17.2.110
Quarles, L. D., Yohay, D. A., Lever, L. W., Caton, R., & Wenstrup, R. J. (1992). Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. Journal of Bone and Mineral Research, 7(6), 683-692. https://doi.org/10.1002/jbmr.5650070613 DOI: https://doi.org/10.1002/jbmr.5650070613
Stanford, C. M., Jacobson, P. A., Eanes, E. D., Lembke, L. A., & Midura, R. J. (1995). Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). Journal of Biological Chemistry, 270(16), 9420-9428. https://doi.org/10.1074/jbc.270.16.9420 DOI: https://doi.org/10.1074/jbc.270.16.9420
Suzuki, H., Tatei, K., Ohshima, N., Sato, S., & Izumi T. (2019). Regulation of MC3T3-E1 differentiation by actin cytoskeleton through lipid mediators reflecting the cell differentiation stage. Biochemical and Biophysical Research Communications, 514(2), 393-400. https://doi.org/10.1016/j.bbrc.2019.04.093 DOI: https://doi.org/10.1016/j.bbrc.2019.04.093
Wang, D., Christensen, K., Chawla, K., Xiao, G., Krebsbach, P. H., & Franceschi, R. T. (1999). Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. Journal of Bone and Mineral Research, 14(6), 893-903. https://doi.org/10.1359/jbmr.1999.14.6.893 DOI: https://doi.org/10.1359/jbmr.1999.14.6.893
Wu, L., & Forsling, W. (1993). Surface complexation of calcium minerals in aqueous solution 2. The complexation of alizarin red S at fluorapatite-water interface. Society for Mining, Metallurgy & Exploration, 11.
Zhang, C., Cao, M., Lan, J., Wei, P., Cai, Q., & Yang, X. (2016). Regulating proliferation and differentiation of osteoblasts on poly(l-lactide)/gelatin composite nanofibers via timed biomineralization. Journal of Biomedical Materials Research Part A, 104(8), 1968-1980. https://doi.org/10.1002/jbm.a.35728 DOI: https://doi.org/10.1002/jbm.a.35728
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Maria Carolina Coelho, Luiz Gabriel Plasier Lazari Guedes de Mello, Sayuri Poli Suguimoto, Roger Rodrigo Fernandes, Karina Fittipaldi Bombonato-Prado

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
Funding data
-
Fundação de Amparo à Pesquisa do Estado de São Paulo
Grant numbers (FAPESP 2021/08029-4)


