Interaction of twenty-two carbazole derivatives with M1-muscarinic receptor using a theoretical model
DOI:
https://doi.org/10.14295/bjs.v3i7.573Keywords:
carbazole, derivatives, M1-muscarinic, receptor, asthma, dockingAbstract
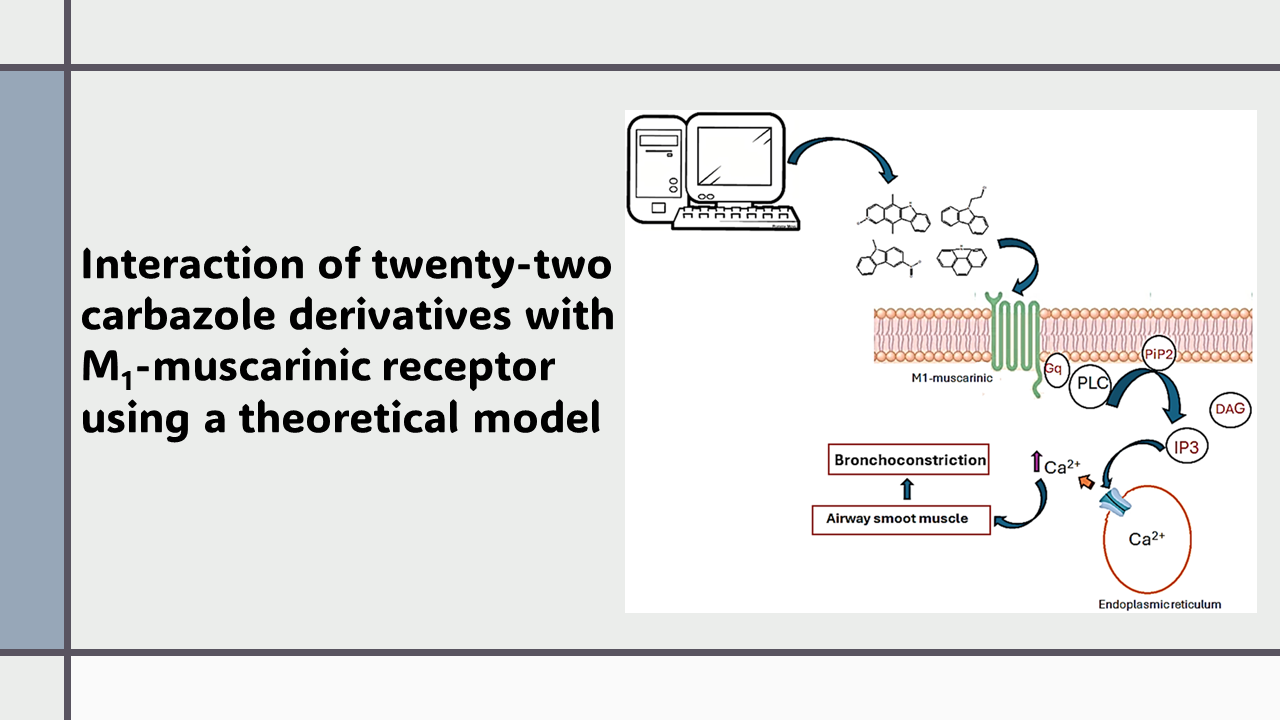
Several drugs have been used to treat asthma diseases, such as salmeterol, ipratropium bromide, montelukast, and fluticasone; however, some of these drugs can cause side effects such as hypokalemia, lactic acidosis, and hypotension. Analyzing these data, this study aimed to evaluate the possible interaction of twenty-two carbazole derivatives with the M1-muscrinic receptor to provide a new therapeutic alternative against asthma. The theoretical interaction of carbazole derivatives with M1-muscrinic receptor surface was determined using 5cxv protein, pirenzepine, atropine, AF-150, and PD159714 drugs as theoretical tools in a DockingServer software. The results showed differences in the interaction of carbazole derivatives with the 5cxv protein surface compared with pyranzepine, atropine, AF-150, and PD159714 drugs. Besides, constant inhibition (Ki) for carbazole derivatives 11 and 22 was lower than for pirenzepine and AF-150 drugs. Other data indicate that Ki values for 11 and 22 were higher than atropine and ipratropium bromide. In addition, the Ki values for compounds 17 and 20 were like both atropine and PD150714 drugs. Finally, Ki values for carbazole derivatives 17 and 20 were lower than pyranzepine, ipratropium bromide, and AF-150 reagents. All these data suggest that carbazole derivatives 11, 17, 20, and 22 may act as M1-muscarinic receptor inhibitor agents; this phenomenon could result in the regulation of bronchial tone in asthma disease.
References
Adkins, J. C., & Brogden, R. N. (1998). Zafirlukast: a review of its pharmacology and therapeutic potential in the management of asthma. Drugs, 55(1), 121-144. https://doi.org/10.2165/00003495-199855010-00008 DOI: https://doi.org/10.2165/00003495-199855010-00008
Augelli-Szafran, C. E., Blankley, C. J., Jaen, J. C., Moreland, D. W., Nelson, C. B., Penvose-Yi, J. R., & Thomas, A. J. (1999). Identification and characterization of m1 selective muscarinic receptor antagonists. Journal of Medicinal Chemistry, 42(3), 356-363. https://doi.org/10.1021/jm980067l DOI: https://doi.org/10.1021/jm980067l
Barrett, P., Bell, B., Cobelli, C., Golde, H., Schumitzky, A., Vicini, P., & Foster, D. (1998). SAAM II: simulation, analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism, 47(4), 484-492. https://doi.org/10.1016/S0026-0495(98)90064-6 DOI: https://doi.org/10.1016/S0026-0495(98)90064-6
Cairns, H., Cox, D., Gould, K. J., Ingall, A., & Suschitzky, J. (1985). New antiallergic pyrano [3, 2-g] quinoline-2, 8-dicarboxylic acids with potential for the topical treatment of asthma. Journal of Medicinal Chemistry, 28(12), 1832-1842. https://doi.org/10.1021/jm00150a014 DOI: https://doi.org/10.1021/jm00150a014
Chabukswar, A., Kuchekar, B., Jagdale, S., Lokhande, P., Chabukswar, V., Shisodia, S., & Ojha, N. (2016). Synthesis and evaluation of analgesic, anti-asthmatic activity of (E)-1-(8-hydroxyquinolin-7-yl)-3-phenylprop-2-en-1 ones. Arabian Journal of Chemistry, 9(5), 704-712. https://doi.org/10.1016/j.arabjc.2014.10.046 DOI: https://doi.org/10.1016/j.arabjc.2014.10.046
Chan, R., & Lipworth, B. (2022). Determinants of asthma control and exacerbations in moderate to severe asthma. The Journal of Allergy and Clinical Immunology: In Practice, 10(10), 2758-2760. https://doi.org/10.1016/j.jaip.2022.06.042 DOI: https://doi.org/10.1016/j.jaip.2022.06.042
Chatkin, J., Correa, L., & Santos, U. (2022). External environmental pollution as a risk factor for asthma. Clinical Reviews in Allergy & Immunology, 62(1), 72-89. https://doi.org/10.1007/s12016-020-08830-5 DOI: https://doi.org/10.1007/s12016-020-08830-5
Colombo, M., Plebani, A., Bosco, A., & Agosti, M. (2022). Severe lactic acidosis and persistent diastolic hypotension following standard dose of intermittent nebulized salbutamol in a child: a case report. Journal of Medical Case Reports, 16(1), 160. https://doi.org/10.1186/s13256-022-03357-z DOI: https://doi.org/10.1186/s13256-022-03357-z
Darveaux, J. I., & Lemanske Jr, R. F. (2014). Infection-related asthma. The Journal of Allergy and Clinical Immunology: In Practice, 2(6), 658-663. https://doi.org/10.1016/S0272-5231(05)70268-9 DOI: https://doi.org/10.1016/j.jaip.2014.09.011
Desai, M., & Oppenheimer, J. J. (2011). Medication adherence in the asthmatic child and adolescent. Current Allergy and Asthma Reports, 11, 454-464. https://doi.org/10.1007/s11882-011-0227-2 DOI: https://doi.org/10.1007/s11882-011-0227-2
Eddershaw, P., Beresford, A., & Bayliss, M. (2000). ADME/PK as part of a rational approach to drug discovery. Drug Discovery Today, 5(9), 409-414. https://doi.org/10.1016/S1359-6446(00)01540-3 DOI: https://doi.org/10.1016/S1359-6446(00)01540-3
El-Khoury, P., Abou Hamad, W., Khalaf, M., El-Hadi, C., Assily, R., Rassi, S., & Khoueir, N. (2023). Ipratropium bromide nasal spray in non‐allergic rhinitis: A systematic review and meta‐analysis. The Laryngoscope, 133(12), 3247-3255. https://doi.org/10.1002/lary.30706 DOI: https://doi.org/10.1002/lary.30706
Figueroa-Valverde, L., Díaz-Cedillo, F., Rosas-Nexticapa, M., Alvarez-Ramirez, M., Mateu-Armad, M., & López-Ramos, M., (2023). Interaction of some amino-nitrile derivatives with vascular endothelial growth factor receptor 1 (VEGFR1) using a theoretical model. Drug Research, 73(06), 355-364. https://doi.org/10.1055/a-2062-3571 DOI: https://doi.org/10.1055/a-2062-3571
Figueroa-Valverde, L., Rosas-Nexticapa, M., Alvarez-Ramirez, M., López-Ramos, M., Díaz-Cedillo, F., & Mateu-Armad, M. V. (2023). Evaluation of biological activity exerted by Dibenzo [b, e] Thiophene-11 (6H)-One on left ventricular pressure using an isolated rat heart model. Drug Research, 73(05), 263-270. https://doi.org/10.1055/a-1995-6351
Figueroa-Valverde, L., Rosas-Nexticapa, M., Alvarez-Ramirez, M., López-Ramos, M., Díaz-Cedillo, F., & Mateu-Armad, M. (2023). Evaluation of biological activity exerted by Dibenzo [b, e] Thiophene-11 (6H)-One on left ventricular pressure using an isolated rat heart model. Drug Research, 73(05), 263-270. https://doi.org/10.1055/a-1995-6351 DOI: https://doi.org/10.1055/a-1995-6351
Fisher, A. (2000). Therapeutic strategies in Alzheimer’s disease: M1 muscarinic agonists. The Japanese Journal of Pharmacology, 84(2), 101-112. https://doi.org/10.1254/jjp.84.101 DOI: https://doi.org/10.1254/jjp.84.101
Friesner, R. A., Banks, J. L., Murphy, R. B., Halgren, T. A., Klicic, J. J., Mainz, D. T., Repasky, M. P., Knoll, E. H., Shelley, M., Perry, J. K., Shaw, D. E., Francis, P., & Shenkin, P. S. (2004). Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of Medicinal Chemistry, 47(7), 1739-1749. https://doi.org/10.1021/jm0306430 DOI: https://doi.org/10.1021/jm0306430
Gaurav, A., & Singh, R. (2014). Pharmacophore modeling, 3DQSAR, and docking-based design of polysubstituted quinolines derivatives as inhibitors of phosphodiesterase 4, and preliminary evaluation of their anti-asthmatic potential. Medicinal Chemistry Research, 12, 5008-5030. https://doi.org/10.1007/s00044-014-1048-3 DOI: https://doi.org/10.1007/s00044-014-1048-3
Gökoğlu, E., Doyuran, B., Özen, G., Duyar, H., Taskin-Tok, T., & Seferoğlu, Z. (2023). Evaluation of the binding properties of a new phenylurea appended carbazole compound to pepsin/trypsin by computational and multi-spectral analysis. Journal of Fluorescence, 1-13. https://doi.org/10.1007/s10895-023-03451-5 DOI: https://doi.org/10.21203/rs.3.rs-3227813/v1
Haarman, M. G., Van-Hunsel, F., & de Vries, T. W. (2017). Adverse drug reactions of montelukast in children and adults. Pharmacology Research & Perspectives, 5(5), e00341. https://doi.org/10.1002/prp2.341 DOI: https://doi.org/10.1002/prp2.341
Henry, L., Ramar, M., Palanisamy, S., Natesan, S., & Kandasamy, R. (2020). Mechanistic investigation of PPARγ-facilitated anti-asthmatic effects of Galangin (Norizalpinin): insights from in silico and in vivo analyses. Biochemical and Biophysical Research Communications, 526(3), 833-840. https://doi.org/10.1016/j.bbrc.2020.03.158 DOI: https://doi.org/10.1016/j.bbrc.2020.03.158
Herrera‐Luis, E., Ortega, V. E., Ampleford, E. J., Sio, Y. Y., Granell, R., de Roos, E., & Pino‐Yanes, M. (2022). Multi‐ancestry genome‐wide association study of asthma exacerbations. Pediatric Allergy and Immunology, 33(6), e13802. https://doi.org/10.1111/pai.13802 DOI: https://doi.org/10.1111/pai.13826
Iwasaki, T., Kondo, K., Kuroda, T., Moritani, Y., Yamagata, S., Sugiura, M., & Ikezawa, K. (1996). Novel selective PDE IV inhibitors as antiasthmatic agents. Synthesis and biological activities of a series of 1-aryl-2, 3-bis (hydroxymethyl) naphthalene lignans. Journal of Medicinal Chemistry, 39(14), 2696-2704. https://doi.org/10.1021/jm9509096 DOI: https://doi.org/10.1021/jm9509096
Jartti, T., & Gern, J. (2017). Role of viral infections in the development and exacerbation of asthma in children. Journal of Allergy and Clinical Immunology, 140(4), 895-906. https://doi.org/10.1016/j.jaci.2017.08.003 DOI: https://doi.org/10.1016/j.jaci.2017.08.003
Jones, G., Willett, P., Glen, R., Leach, A., & Taylor, R. (1997). Development and validation of a genetic algorithm for flexible docking. Journal of Molecular Biology, 267, 727-748. https://doi.org/10.1006/jmbi.1996.0897 DOI: https://doi.org/10.1006/jmbi.1996.0897
Koumpagioti, D., Moriki, D., Boutopoulou, B., Matziou, V., Loukou, I., Priftis, K., & Douros, K. (2023). The association between CFTR gene mutation heterozygosity and asthma development: A systematic review. Journal of Clinical Medicine, 12(6), 2403. https://doi.org/10.3390/jcm12062403 DOI: https://doi.org/10.3390/jcm12062403
Kraft, M. (2000). The role of bacterial infections in asthma. Clinics in Chest Medicine, 21(2), 301-313. DOI: https://doi.org/10.1016/S0272-5231(05)70268-9
Lauro, F., Marcela, R., Maria, L., Magdalena, A., Virginia, M., Francisco, D., & Montserrat, M. (2022). Evaluation of biological activity of a diazocine derivative against heart failure using an ischemia-reperfusion injury model. Drug Research, 72(07), 404-411. https://doi.org/10.1055/a-1840-3199 DOI: https://doi.org/10.1055/a-1840-3199
Lee, J., Kim, J., Choi, J., & Na, J. (2022). Respiratory reviews in asthma 2022. Tuberculosis and Respiratory Diseases, 85(4), 283. https://doi.org/10.4046/trd.2022.0097 DOI: https://doi.org/10.4046/trd.2022.0097
Li, X., Zhang, Y., Zhang, R., Chen, F., Shao, L., & Zhang, L. (2022). Association between e-cigarettes and asthma in adolescents: a systematic review and meta-analysis. American Journal of Preventive Medicine, 62(6), 953-960. https://doi.org/10.1016/j.amepre.2022.01.015 DOI: https://doi.org/10.1016/j.amepre.2022.01.015
Liu, Y., Qu, H. Q., Qu, J., Chang, X., Mentch, F., Nguyen, K., & Hakonarson, H. (2022). Burden of rare coding variants reveals genetic heterogeneity between obese and non-obese asthma patients in the African American population. Respiratory Research, 23(1), 116. https://doi.org/10.1186/s12931-022-02039-0 DOI: https://doi.org/10.1186/s12931-022-02039-0
Marques, L., & Vale, N. (2022). Salbutamol in the management of asthma: A review. International Journal of Molecular Sciences, 23(22), 14207. https://doi.org/10.3390/ijms232214207 DOI: https://doi.org/10.3390/ijms232214207
Mikhail, I., & Grayson, M. H. (2019). Asthma and viral infections: An intricate relationship. Annals of Allergy, Asthma & Immunology, 123(4), 352-358. https://doi.org/10.1016/j.anai.2019.06.020 DOI: https://doi.org/10.1016/j.anai.2019.06.020
Nayak, A. (2004). A review of montelukast in the treatment of asthma and allergic rhinitis. Expert Opinion on Pharmacotherapy, 5(3), 679-686. https://doi.org/10.1517/14656566.5.3.679 DOI: https://doi.org/10.1517/14656566.5.3.679
Österberg, F., Morris, G., Sanner, M., Olson, A., & Goodsell, D. (2002). Automated docking to multiple target structures: incorporation of protein mobility and structural water heterogeneity in AutoDock. Proteins, 46, 34-40. https://doi.org/10.1002/prot.10028 DOI: https://doi.org/10.1002/prot.10028
Patel, M., Pandey, N., Timaniya, J., Parikh, P., Chauhan, A., Jain, N., & Patel, K. (2021). Coumarin–carbazole based functionalized pyrazolines: Synthesis, characterization, anticancer investigation and molecular docking. RSC Advances, 11(44), 27627-27644. https://doi.org/10.1039/D1RA03970A DOI: https://doi.org/10.1039/D1RA03970A
Rarey, M., Kramer, B., Lengauer, T., & Klebe, G. (1996). A fast flexible docking method using an incremental construction algorithm. Journal of Molecular Biology, 261, 470-489. https://doi.org/doi:10.1006/jmbi.1996.0477 DOI: https://doi.org/10.1006/jmbi.1996.0477
Remko, M. (2007). Acidity, lipophilicity, solubility, absorption, and polar surface area of some ACE inhibitors. Chemical Papers, 61, 133-141. https://doi.org/10.2478/s11696-007-0010-y DOI: https://doi.org/10.2478/s11696-007-0010-y
Reyes-Angel, J., Kaviany, P., Rastogi, D., & Forno, E. (2022). Obesity-related asthma in children and adolescents. The Lancet Child & Adolescent Health, 6(10), 713-724. https://doi.org/10.1016/S2352-4642(22)00185-7 DOI: https://doi.org/10.1016/S2352-4642(22)00185-7
Rodrigo, G., Rodrigo, C., & Hall, J. (2004). Acute asthma in adults: a review. Chest, 125(3), 1081-1102. https://doi.org/10.1378/chest.125.3.1081 DOI: https://doi.org/10.1378/chest.125.3.1081
Sabnis, R. (2022). Novel IRAK4 inhibitors for treating asthma. ACS Medicinal Chemistry Letters, 13(8), 1219-1220. https://doi.org/10.1021/acsmedchemlett.2c00324 DOI: https://doi.org/10.1021/acsmedchemlett.2c00324
Sandham, D. A., Barker, L., Brown, L., Brown, Z., Budd, D., Charlton, S., & Willis, J. (2017). Discovery of fevipiprant (NVP-QAW039), a potent and selective DP2 receptor antagonist for treatment of asthma. ACS Medicinal Chemistry Letters, 8(5), 582-586. https://doi.org/10.1021/acsmedchemlett.7b00157 DOI: https://doi.org/10.1021/acsmedchemlett.7b00157
Scheinin, M., Koulu, M., Laurikainen, E., & Allonen, H. (1987). Hypokalaemia and other non‐bronchial effects of inhaled fenoterol and salbutamol: a placebo‐controlled dose‐response study in healthy volunteers. British Journal of Clinical Pharmacology, 24(5), 645-653. https://doi.org/10.1111/j.1365-2125.1987.tb03224.x DOI: https://doi.org/10.1111/j.1365-2125.1987.tb03224.x
Shaikh, S., Dhavan, P., Singh, P., Uparkar, J., Vaidya, S. P., Jadhav, B. L., & Ramana, M. V. (2022). Synthesis of carbazole based α-aminophosphonate derivatives: design, molecular docking and in vitro cholinesterase activity. Journal of Biomolecular Structure and Dynamics, 40(11), 4801-4814.
https://doi.org/10.1080/07391102.2020.1861981 DOI: https://doi.org/10.1080/07391102.2020.1861981
Stikker, B., Hendriks, R., & Stadhouders, R. (2023). Decoding the genetic and epigenetic basis of asthma. Allergy, 78(4), 940-956. https://doi.org/10.1111/all.15666 DOI: https://doi.org/10.1111/all.15666
Struckmann, N., Schwering, S., Wiegand, S., Gschnell, A., Yamada, M., Kummer, W., & Haberberger, R. (2003). Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Molecular Pharmacology, 64(6), 1444-1451. https://doi.org/10.1124/mol.64.6.1444 DOI: https://doi.org/10.1124/mol.64.6.1444
Svedmyr, N. (1985). Fenoterol: A Beta2‐adrenergic Agonist for Use in Asthma; Pharmacology, Pharmacokinetics, Clinical Efficacy and Adverse Effects. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 5(3), 109-126. https://doi.org/10.1002/j.1875-9114.1985.tb03409.x DOI: https://doi.org/10.1002/j.1875-9114.1985.tb03409.x
Thal, D., Sun, B., Feng, D., Nawaratne, V., Leach, K., Felder, C, & Christopoulos, A. (2016). Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature, 531(7594), 335-340. DOI: https://doi.org/10.1038/nature17188
Toropov, A., Toropova, A., Marzo, M., Dorne, J., Georgiadis, N., & Benfenati, E. (2017). QSAR models for predicting acute toxicity of pesticides in rainbow trout using the CORAL software and EFSA’s OpenFoodTox database. Environmental Toxicology and Pharmacology, 53, 158-163. https://doi.org/10.1016/j.etap.2017.05.011 DOI: https://doi.org/10.1016/j.etap.2017.05.011
Ukita, T., Sugahara, M., Terakawa, Y., Kuroda, T., Wada, K., Nakata, A., & Naito, K. (1999). Novel, potent, and selective phosphodiesterase-4 inhibitors as antiasthmatic agents: synthesis and biological activities of a series of 1-pyridylnaphthalene derivatives. Journal of Medicinal Chemistry, 42(6), 1088-1099. https://doi.org/10.1021/jm980314l DOI: https://doi.org/10.1021/jm980314l
Vedani, A., Dobler, M., Hu, Z., & Smieško, M. (2015). OpenVirtualToxLab-a platform for generating and exchanging in silico toxicity data. Toxicology Letters, 232(2), 519-532. https://doi.org/10.1016/j.toxlet.2014.09.004 DOI: https://doi.org/10.1016/j.toxlet.2014.09.004
Venkatachalam, C., Jiang, X., Oldfield, T., & Waldman, M. (2003). LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. Journal Molecular Graphics and Modelling, 21, 289-307. https://doi.org/10.1016/S1093-3263(02)00164-X DOI: https://doi.org/10.1016/S1093-3263(02)00164-X
Venkatapathy, R., Moudgal, C., & Bruce, R. (2004). Assessment of the oral rat chronic lowest observed adverse effect level model in TOPKAT, a QSAR software package for toxicity prediction. Journal of Chemical Information and Computer Sciences, 44(5), 1623-1629. https://doi.org/10.1021/ci049903s DOI: https://doi.org/10.1021/ci049903s
Wheatley, L., Holloway, J., Svanes, C., Sears, M., Breton, C., Fedulov, A., & Arshad, S. (2022). The role of epigenetics in multi‐generational transmission of asthma: An NIAID workshop report‐based narrative review. Clinical & Experimental Allergy, 52(11), 1264-1275. https://doi.org/10.1111/cea.14223 DOI: https://doi.org/10.1111/cea.14223
Widzowski, D., Wu, E., & Helander, H. (1997). Selective muscarinic M1 antagonists: drug design and discovery. Drug Discovery Today, 2(8), 341-350. https://doi.org/10.1016/S1359-6446(97)01076-3 DOI: https://doi.org/10.1016/S1359-6446(97)01076-3
Xing, G., Li, D., Woo, A., Zhi, Z., Ji, L., Xing, R., & Cheng, M. (2022). Discovery of a highly selective β2-adrenoceptor agonist with a 2-amino-2-phenylethanol scaffold as an oral antiasthmatic agent. Journal of Medicinal Chemistry, 65(7), 5514-5527. https://doi.org/10.1021/acs.jmedchem.1c02006 DOI: https://doi.org/10.1021/acs.jmedchem.1c02006
Yang, Y., Engkvist, O., Llinàs, A., & Chen, H. (2012). Beyond size, ionization state, and lipophilicity: influence of molecular topology on absorption, distribution, metabolism, excretion, and toxicity for druglike compounds. Journal of Medicinal Chemistry, 55(8), 3667-3677. https://doi.org/10.1021/jm201548z DOI: https://doi.org/10.1021/jm201548z
Zlotos, D., Bender, W., & Holzgrabe, U. (1999). Muscarinic receptor agonists and antagonists. Expert Opinion on Therapeutic Patents, 9(8), 1029-1053. https://doi.org/10.1517/13543776.9.8.1029 DOI: https://doi.org/10.1517/13543776.9.8.1029
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Lauro Figueroa-Valverde, Maria López-Ramos, Marcela Rosas-Nexticapa, Magdalena Alvarez-Ramirez, Maria Virginia Mateu-Armad, Lenin Hau-Heredia, Regina Cauich-Carrillo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


