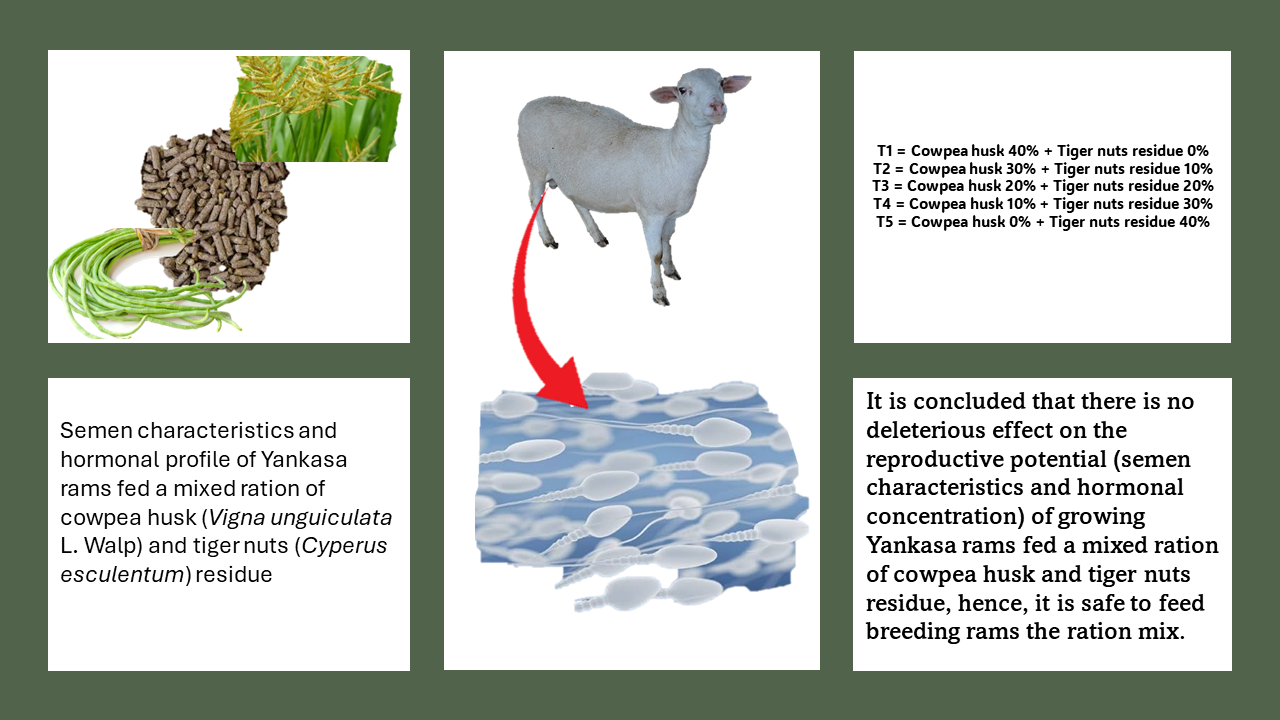
Semen characteristics and hormonal profile of Yankasa rams fed a mixed ration of cowpea husk (Vigna unguiculata L. Walp) and tiger nuts (Cyperus esculentum L.) residue
DOI:
https://doi.org/10.14295/bjs.v3i6.570Keywords:
semen, hormone, Yankasa rams, cowpea husk, tiger nut residueAbstract
Thirty growing and healthy Yankasa rams were randomly allotted five dietary treatments with six animals per treatment to ascertain the effect of diet on their reproductive potential and hormonal profile. Treatments compared were T1 (cowpea husk 40% + tiger nuts residue 0%), T2 (cowpea husk 30% + tiger nuts residue 10%), T3 (cowpea husk 20% + tiger nuts residue 20%), T4 (cowpea husk 10% + tiger nuts residue 30%), and T5 (cowpea husk 0% + tiger nuts residue 40%). The results of the chemical composition of the diets showed that the dry matter was high for all treatments. The semen characteristics and hormonal profile showed significant differences (p < 0.05) in all the parameters observed but were within normal ranges. However, the group fed a high percent tiger nut mixed ratio had a depreciating effect on semen characteristics, and LH, FSH, and testosterone levels. There is no deleterious effect on the reproductive potential of growing Yankasa rams fed varying levels of cowpea husk and tiger nut residue at the inclusion levels in this study. Hence, it is safe to feed breeding rams the ration mix. However, the authors advise caution of including tiger nuts levels of up to 40% in a mixed ration. further investigation may be conducted with ewes to determine the effect of a mixed ration of cowpea husk and tiger nut residue in reproduction.
References
Abaje, I. B., Achiebo, P. J., & Matazu, B. M. (2018). Spatio-temporal analysis of rainfall distribution in Kaduna state. Ghana Journal of Geology, 10(1), 1-21. https://www.ajol.info/index.php/gjg/article/view/170403
Abaje, I. B., Sawa, B. A., Iguisi, E. O., & Ibrahim, A. A. (2016). Impacts of climate change and adaptation strategies in rural communities of Kaduna State, Nigeria. Ethiopian Journal of Environmental Studies and Management, 9(1), 97-108. https://doi.org/10.4314/ejesm.v9i1.9 DOI: https://doi.org/10.4314/ejesm.v9i1.9
Abdu, S. B., Ahmed, H., Jokthan, M. R., Adamu, H. Y., & Yashim, S. M. (2012). Effect of levels of Ficus (Ficus cyncomorus) supplementation on voluntary feed intake, nutrient digestibility and nitrogen in Yankasa bucks fed Urea treated maize stover basal diet. Iranian Journal of Animal Science, 2(2), 151-155. https://sanad.iau.ir/journal/ijas/Article/513551?jid=513551
Abubakar, M. S., Mohammed, A., & Bosso, N. A. (2014). Semen quality assessment in Yankasa rams: effects of age, breed, and season. Journal of Veterinary Medicine, 2014, 926836.
Adebayo, A. O., Adeyemi, O. A., & Olorede, B. R. (2014). Semen characteristics of Yankasa rams fed diets supplemented with different levels of soya bean meal. Journal of Animal Science and Advancements, 4(5), 755-761.
Akinlade, J. O., Ojedapo, L. O., & Bamgbose, A. M. (2020). Semen characteristics and haemato-biochemical indices of
Yankasa rams fed graded levels of water-soaked wild sunflower (Tithonia diversifolia) as replacement for maize offal. Tropical Animal Health Production, 52(5), 2457-2466.
Al-Dujaili, E. A. S., Hajleh, M. N. A., & Chalmers, R. (2020). Effects of ginseng ingestion on salivary testosterone and DHEA levels in healthy females: An exploratory study. Nutrients, 12(6), 1582. https://doi.org/10.3390/nu12061582 DOI: https://doi.org/10.3390/nu12061582
Allouh, M. Z., Daradka, H. M., & Abu-Ghaida, J. H. (2015). Influence of Cyperus esculentus tubers (tiger nut) on male rat copulatory behavior. BMC Complementary and Alternative Medicine, 15, 331. https://doi.org/10.1186/s12906-015-0851-9 DOI: https://doi.org/10.1186/s12906-015-0851-9
Al-Samarrae, S. H. (2009). Semen quality of arrabi and karradi iraqi rams. Diyala Agricultural Science Journal, 1(2), 30-36. http://148.72.244.84:8080/xmlui/handle/xmlui/11417
AOAC. (2002). Official Methods of Analysis 16th edition. Association of Official Analytical Chemists, Washington, DC.
Archibong, E. E., Nsa, E. E., & Umoren, U. E. (2018). Nutritional evaluation of tigernut (Cyperus esculentus) meal as a replacement for maize in broiler diets. Nigerian Journal of Animal Production, 45(4), 90-98. https://doi.org/10.51791/njap.v45i4.541 DOI: https://doi.org/10.51791/njap.v45i4.541
Ax, R. L., Dally, M. R., Didon, B. A., Lenz, R. W., Love, C. C., & Varner, D. D. (2000). Artificial insemination. In: Hafez, B., & Hafez, E. S. E. Reproduction in farm animals. 7th ed. Philadelphia: Lea and Febinger, 376-389 p. DOI: https://doi.org/10.1002/9781119265306.ch26
Ayim-Akonor, M., Fuseini, A., & Boateng, M. (2015). Performance and seminal characteristics of West African dwarf rams fed varying levels of cowpea husk and bovine blood meal mixture. Journal of Agricultural Science and Technology, 17(1), 63-73.
Azizunnesa, K., Zohara, B. F., Bari, F. Y., & Alam, M. G. S. (2014). Baseline study of reproductive performances of indigenous rams in Bangladesh. IOSR Journal of Agriculture and Veterinary Science, 7(6), 83-89. DOI: https://doi.org/10.9790/2380-07618389
Azizunnesa, K., Zohara, B.F., Bari, F.Y. & Alam, M.G.S. (2013) Effects of concentrate supplementation on reproductive performances and semen quality of indigenous rams in Bangladesh. Journal of Embryo Transfer, 28(4), 325-335. https://doi.org/10.12750/JET.2013.28.4.325 DOI: https://doi.org/10.12750/JET.2013.28.4.325
Carlos, P., Alvarez, S., Martin-Gil, J., & Abecia, J.A. (2016). Plasma hormonal levels of rams are affected by sexual activity and confinement in a semen collection centre. ARC Journal of Animal and Veterinary Sciences, 2(2), 15-21. DOI: https://doi.org/10.20431/2455-2518.0202002
Carneiro da Silva, A., Costa Santos, D., Lopes Teixeira Junior, D., Bento da Silva, P., Cavalcante dos Santos, R., & Siviero, A. (2019). Cowpea: A strategic legume species for food security and health. IntechOpen. https://doi.org/10.5772/intechopen.79006 DOI: https://doi.org/10.5772/intechopen.79006
Chen, Y-C., Nagpal, M. L., Stocco, D. M., & Lin, T. (2007). Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. Journal of Endocrinology, 192(3), 527-37. https://doi.org/10.1677/JOE-06-0087 DOI: https://doi.org/10.1677/JOE-06-0087
Chenoweth, P. J. (2005). Genetic sperm defect. Theriogenology, 64(3), 457-468. https://doi.org/10.1016/j.theriogenology.2005.05.005 DOI: https://doi.org/10.1016/j.theriogenology.2005.05.005
Colas, G. (1983). Factors affecting the quality of ram semen. In: Haresign, W. (Ed.), Sheep Production. Butterworths, London, 453-465 p.
Enyiukwu, D. N., Amadioha, A. C., & Ononuju, C. C. (2018). Nutritional significance of cowpea leaves for human consumption. Greener Trends in Food Science and Nutrition, 1(1), 01-10. http://doi.org/10.15580/GTFSN.2018.1.061818085 DOI: https://doi.org/10.15580/GTFSN.2018.1.061818085
Estienne, M. J., Riesen, J. W. & Whitacre, M. D. (1993). Relationships among sexual behavior, age, and peripheral testosterone concentrations in pubertal Suffolk rams. Journal of Animal Science, 71(2), 343-348.
Garba, M. G., Dayyabu, S. K., Gaddafi, S. Aruwayo, A., & Salisu, S. U. (2022). Effect of graded levels of bitter leaf (Vernonia amygdalin) on performance and semen characteristics of Yankasa rams in Sudan Savannah zone Nigeria. International Journal of Science Academic Research, 03(06), 3905-3909.
Hantzopoulou, G-C., Sawyer, G., Tilbrook, A., & Narayan, E. (2022). Intra-and inter-sample variation in wool cortisol concentrations of Australian Merino lambs between twice or single shorn ewes. Frontiers in Animal Science, 3, 890914. https://doi.org/10.3389/fanim.2022.890914 DOI: https://doi.org/10.3389/fanim.2022.890914
Ihukwumere, F. C., & Okere, C. (1990). Effects of frequent ejaculations on semen characteristics of Nigerian Yankasa rams. Small Ruminant Research, 3(1), 77-83. https://doi.org/10.1016/0921-4488(90)90034-4 DOI: https://doi.org/10.1016/0921-4488(90)90034-4
Jha, P. K., Alam, G. S., Al Mansur, A., Islam, T., & Bari, Y. F. (2018). Selection of breeding rams by evaluating semen quality. Journal of Applied Animal Science, 11(1), 9-20.
Kaya, A., Aksoy, M., & Tekeli, T. (2002). Influence of ejaculation frequency on sperm characteristics, ionic composition, and enzymatic activity of seminal plasma in rams. Small Ruminant Research, 44(2), 153-158. https://doi.org/10.1016/S0921-4488(02)00051-2 DOI: https://doi.org/10.1016/S0921-4488(02)00051-2
Khalifa, T., Lymberopoulos, A., & Theodosiadou, E. (2013). Association of soybean-based extenders with field fertility of stored ram (Ovis aries) semen: a randomized double-blind parallel group design. Theriogenology, 79(3), 517-27. https://doi.org/10.1016/j.theriogenology.2012.11.009 DOI: https://doi.org/10.1016/j.theriogenology.2012.11.009
Madhuri, D., Gupta, V., Nema, S., Patidar, A., Shivhare, M., & Singh, N. (2012). Modern semen evaluation techniques in domestic animals: A review. DHR International Journal of Biomedical Life Sciences, 3(1), 62-83.
Malama, E., Bollwein, H., Taitzoglou, I. A., Theodosiou, T., Boscos, C. M., & Kiossis, E. (2013). Chromatin integrity of ram spermatozoa. Relationships to annual fluctuations of scrotal surface temperature and temperature-humidity index. Theriogenology, 80(5), 533-541. https://doi.org/10.1016/j.theriogenology.2013.05.019 DOI: https://doi.org/10.1016/j.theriogenology.2013.05.019
McNatty, K. P., Isaacs, K. L., Gentle, L., Berry, L., Hudson, N. L., Young, W., McLeod, B. J. (1998). Bioactive and immunoreactive FSH concentrations in ewe and ram lambs over the first year of life. Animal Reproduction Science, 51(2), 155-166. https://doi.org/10.1016/S0378-4320(98)00055-4 DOI: https://doi.org/10.1016/S0378-4320(98)00055-4
Meissner, H. H, Viij, M. D., & Van Neierkeki, W. A. (1991). Intake and digestibility of sheep of anthephora, panicum rhode and smuts finger grass pastures. In: Proceedings of the 4th International Rangeland Congress, September, Montpelier, France, 1991, 648-649.
Micallef, J. V., Hayes, M. M., Latif, A., Ahsan, R., & Sufi, S. B. (1995). Serum binding of steroid tracers and its possible effects on direct steroid immunoassay. Annals of Clinical Biochemistry, 32(Pt 6), 566-574. https://doi.org/10.1177/000456329503200609 DOI: https://doi.org/10.1177/000456329503200609
Minson, D. J. (1990). Forage in ruminant nutrition. Academic Press, San Diego, USA, 483-484 p.
Mozo, R., Galeote, A. I., Alabart, J. L., Enrique, F., & José F. (2015). Evaluating the reproductive ability of breeding rams in North-Eastern Spain using clinical examination of the body and external genitalia. BMC Veterinary Research, 11, 289. https://doi.org/10.1186/s12917-015-0600-9 DOI: https://doi.org/10.1186/s12917-015-0600-9
Nabil, A. H., Sayed, T. I., & Marzook, M. A. (2006). Artificial Insemination. In: Artificial Insemination and Embryo Transfer in Farm Animals. 1st ed. King Faisal University: Publisher King Fahd National Library, 55-113 p.
Nagatani, S., Zeng, Y., Keisler, D. H., Foster, D. L., & Jaffe, C. A. (2000). Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in sheep. Endocrinology, 141(11), 3965-3975. https://doi.org/10.1210/endo.141.11.7762 DOI: https://doi.org/10.1210/en.141.11.3965
Nasir, M., Njidda, A. A., & Hassan, A. M. (2014). Testicular histometry, gonadal and extra gonadal sperm reserve of Red Sokoto Bucks fed cotton seed cake. Journal of Science, 4(4), 227-232.
Njidda, A. A., Zikachat, S. S., & Udokpoh, P. R (2018). Effect of substituting soya bean meal with Gmelina arborea leaf meal on performance and rumen fermentation of Red Sokoto Bucks. Nigerian Agricultural Journal, 49(2), 294-301. https://www.ajol.info/index.php/naj/article/view/189492
O’Shaughnessy, P. J. (2014). Hormonal control of germ cell development and spermatogenesis. Seminars in Cell and Development Biology, 29, 55-65. https://doi.org/10.1016/j.semcdb.2014.02.010 DOI: https://doi.org/10.1016/j.semcdb.2014.02.010
Okafor, E. C., Lakpini, C. A. M., & Fayomi, A. (2012). Dried gmelina (Gmelina arborea roxb) leaves as replacement forage to groundnut haulms in the diet of fattening Red Sokoto bucks. International Journal of Agriculture and Biosciences, 1(1), 5-10. https://www.ijagbio.com/wp-content/uploads/pdf-files/Volume-1-Issue-1-2012/5-10.pdf
Olafadehan, O. A., Okunade, S. A., Kholif, A. E., Oludare, T. R., Okoye, G. C., Onwih, E. E., Anaso, E. U., Gero, A., & Shoyombo, A. J. (2022). Urea-amoniated cowpea husk in goat diet: Feed utilization, ruminal fermentation, nutrient digestibility, nitrogen utilization, blood metabolites and growth performance. Research Square, 1. https://doi.org/10.21203/rs.3.rs-1753258/v1 DOI: https://doi.org/10.21203/rs.3.rs-1753258/v1
Olsen, R. L., Schanbacher, B. D., & Barnes, R. J. (1985). Plasma concentrations of follicle-stimulating hormone and testosterone in rams during the breeding season. Journal of Animal Science, 61(12), 3979-3984.
Osinowo, O. A., Ahmed, M. S., & Ekpe, G. A. (1988). Semen quality and sperm output of Yankasa rams at different ages. Theriogenology, 29(2), 381-386. https://doi.org/10.1016/0093-691X(88)90241-5 DOI: https://doi.org/10.1016/0093-691X(88)90241-5
SAS. (Statistical Analysis Systems) (2005). Statistical Package for Analysis (10.0). Statistical Analysis System Institute, Cary.
Schanbacher, B. D. (1989). The physiology of reproduction in the ram. Journal of Animal Science, 68(1), 1-24.
Silva, M. J. B., Monteiro, P. L. J., Ferreira, A. M., Mestre, P. R. A., & Madeira, M. J. (2008). Testosterone levels in peripheral blood and seminal plasma of mature and young rams. Revista Brasileira de Zootecnia, 37(10), 1833-1838.
Toe, F., Rege, J. E. O., Mukasa-Mugerwa, E., Tembely, S., Anindo, D., & Baker, R. L. (2000). Reproductive characteristics of Ethiopian highland sheep I.: genetic parameters of testicular measurements in ram lambs and relationship with age at puberty in ewe lambs. Small Ruminant Research, 36(3), 227-240. https://doi.org/10.1016/S0921-4488(99)00117-0 DOI: https://doi.org/10.1016/S0921-4488(99)00117-0
Umapathy E. (1992). Diet-induced changes in the male reproductive system of rats. Archives of Andrology, 29(1), 49-58. https://doi.org/10.3109/01485019208987708 DOI: https://doi.org/10.3109/01485019208987708
Van Soest, P. J., Robertson, J. B., & Lewi, B. A. (1991). Methods for dietary fibre, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74(10), 3583-3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2 DOI: https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Yue, D., Yan, L., Luo, H., Xu, X., & Jin, X. (2010). Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Animal Reproductive Science, 118(2-4), 217-222. https://doi.org/10.1016/j.anireprosci.2009.08.004 DOI: https://doi.org/10.1016/j.anireprosci.2009.08.004
Zhao, W., Jing, J., Shao, Y., Zeng, R., Cencen Wang, C., Yao, B., & Hang D. (2020). Circulating sex hormone levels in relation to male sperm quality. BMC Urology, 20, 101. https://doi.org/10.1186/s12894-020-00674-7 DOI: https://doi.org/10.1186/s12894-020-00674-7
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Ahmed Amin Njidda, Isaac Sammani Butswat, Hosea Yakubu, Ijeoma Chika Chibuogwu, Abayomi Samuel Bankole

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


