Theoretical interaction of muscarinic receptor antagonist with vascular endothelial growth factor receptors (VEGF-R1, R2 and R3) as a therapeutic alternative to treat cancer
DOI:
https://doi.org/10.14295/bjs.v4i5.734Keywords:
cancer, muscarinic, antagonists, angiogenesisAbstract
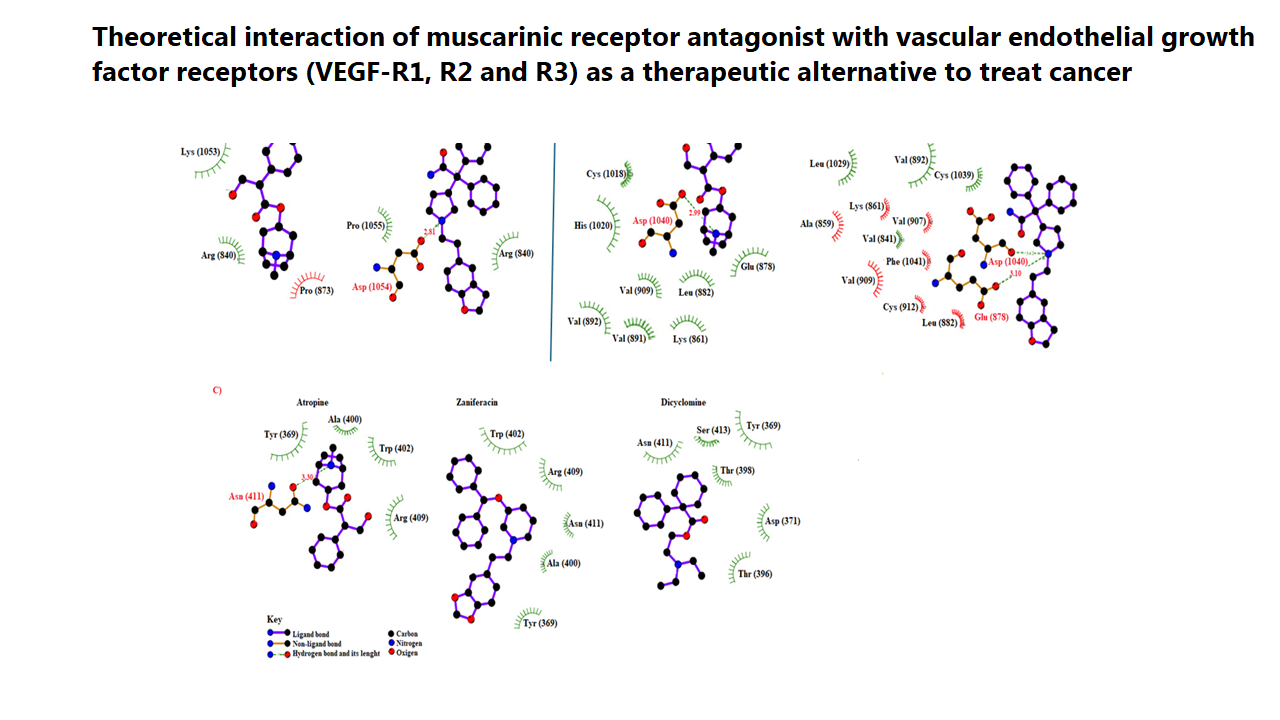
Several biomolecules have been the target of some drugs for the treatment of cancer; however, there is little information on the interaction of muscarinic antagonists with vascular endothelial growth factor receptor (VEGF-R1, R2, R3). The aim of this research was to determine the possible interaction of muscarinic antagonists such as atropine, ML381, af-dx 386, azaprophen, darifenacin, dicyclomine, PD-102807, pirenzepine, telenzepine, Zamifenacin, and cyclohexylamine with VEGF-R1, R2, and R3. The theoretical interaction of muscarinic antagonists with VEGF-R1, R2, and R3 was carried out using the 2ho4, 3hng, and 4bsj proteins as theoretical tools. Besides, cabozantinib, pazopanib, regorafenib, and sorafenib drugs were used as controls. The results showed differences in the number of aminoacid residues and energy levels involved in the interaction of muscarinic antagonists with 2ho4, 3hng, and 4bsj proteins compared with the controls. Besides, the inhibition constants (Ki) values for atropine, ML-381, zaniferacin, and dicyclomine were lower compared with some controls. In conclusion, the results suggest that atropine, ML-381, zaniferacin, and dicyclomine could act as VEGF receptor inhibitors, could result in changes in the biological activity of angiogenesis, and this phenomenon could be translated as a decrease in cancer cell growth. Therefore, these drugs could be a good therapeutic alternative to evaluate their biological activity in some cancer models.
References
Ahmad, A., & Nawaz, M. I. (2022). Molecular mechanism of VEGF and its role in pathological angiogenesis. Journal of cellular biochemistry, 123(12), 1938-1965. https://doi.org/10.1002/jcb.30344 DOI: https://doi.org/10.1002/jcb.30344
Ali, A., Jadhav, A., Jangid, P., Patil, R., Shelar, A., & Karuppayil, S. M. (2018). The human muscarinic acetylcholine receptor antagonist, Dicyclomine targets signal transduction genes and inhibits the virulence factors in the human pathogen, Candida albicans. The Journal of Antibiotics, 71(4), 456-466.
Ali, A., Jadhav, A., Jangid, P., Patil, R., Shelar, A., & Karuppayil, S. M. (2018). The human muscarinic acetylcholine receptor antagonist, Dicyclomine targets signal transduction genes and inhibits the virulence factors in the human pathogen, Candida albicans. The Journal of Antibiotics, 71(4), 456-466. DOI: https://doi.org/10.1038/s41429-017-0013-z
Anbarasu, S., & Anbarasu, A. (2023). Cancer-biomarkers associated with sex hormone receptors and recent therapeutic advancements: a comprehensive review. Medical Oncology, 40(6), 171. DOI: https://doi.org/10.1007/s12032-023-02044-3
Bikadi, Z., & Hazai, E. (2009). Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. Journal of cheminformatics, 1, 1-16. DOI: https://doi.org/10.1186/1758-2946-1-15
Bourne, D. W. (1997). Using the Internet as a pharmacokinetic resource. Clinical pharmacokinetics, 33(3), 153-160. DOI: https://doi.org/10.2165/00003088-199733030-00001
Brave, S. R., Ratcliffe, K., Wilson, Z., James, N. H., Ashton, S., Wainwright, A., ... & Barry, S. T. (2011). Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Molecular cancer therapeutics, 10(5), 861-873. https://doi.org/10.1158/1535-7163.MCT-10-0976 DOI: https://doi.org/10.1158/1535-7163.MCT-10-0976
Carroll, F. I., Abraham, P., Mascarella, S. W., Singh, P., Moreland, C. G., Sankar, S. S., & Triggle, D. J. (1991). Crystal, solution, and molecular modeling structural properties and muscarinic antagonist activity of azaprophen. Journal of medicinal chemistry, 34(4), 1436-1440. https://doi.org/10.1021/jm00108a030
Carroll, F. I., Abraham, P., Mascarella, S. W., Singh, P., Moreland, C. G., Sankar, S. S., ... & Triggle, D. J. (1991). Crystal, solution, and molecular modeling structural properties and muscarinic antagonist activity of azaprophen. Journal of medicinal chemistry, 34(4), 1436-1440. https://doi.org/10.1021/jm00108a030 DOI: https://doi.org/10.1021/jm00108a030
Cazzola, M., Matera, M. G., Liccardi, G., Sacerdoti, G., D'Amato, G., & Rossi, F. (1994). Effect of telenzepine, an M1-selective muscarinic receptor antagonist, in patients with nocturnal asthma. Pulmonary Pharmacology, 7(2), 91-97. https://doi.org/10.1006/pulp.1994.1010 DOI: https://doi.org/10.1006/pulp.1994.1010
Chen, J., Shin, V. Y., Cheuk, W. Y. I., Siu, J. M. T., & Kwong, A. (2020). Cholinergic receptor muscarinic 3 (CHRM3) contributes to breast cancer tumorigenesis through angiogenesis regulation. In San Antonio Breast Cancer Symposium (SABCS). American Association Cancer Research, 80(4), 1-7. http://dx.doi.org/10.1158%2F1538- DOI: https://doi.org/10.1158/1538-7445.SABCS19-P1-07-01
SABCS19-P1-07-01
Chhikara, B. S., & Parang, K. (2023). Global Cancer Statistics 2022: the trends projection analysis. Chemical Biology Letters, 10(1), 451-451.
Cossy, J., Dumas, C., & Pardo, D. G. (1997). A short and efficient synthesis of zamifenacin a muscarinic M3 receptor antagonist. Bioorganic & Medicinal Chemistry Letters, 7(10), 1343-1344. https://doi.org/10.1016/S0960-894X(97)00221-7 DOI: https://doi.org/10.1016/S0960-894X(97)00221-7
Costa, M. F. F. D., Bilobran, M. A., de Oliveira, L. C., Muniz, A. H. R., Chelles, P. A., & Sampaio, S. G. D. S. M. (2024). Correlation between cancer pain and quality of life in patients with advanced cancer admitted to a palliative care unit. American Journal of Hospice and Palliative Medicine®, 41(8), 882-888. https://doi.org/10.1177/10499091231195318 DOI: https://doi.org/10.1177/10499091231195318
Eguchi, R., Kawabe, J. I., & Wakabayashi, I. (2022). VEGF-independent angiogenic factors: beyond VEGF/VEGFR2 signaling. Journal of Vascular Research, 59(2), 78-89. https://doi.org/10.1159/000521584 DOI: https://doi.org/10.1159/000521584
El‐Helby, A. G. A., Ayyad, R. R., Sakr, H., El‐Adl, K., Ali, M. M., & Khedr, F. (2017). Design, synthesis, molecular docking, and anticancer activity of phthalazine derivatives as VEGFR‐2 inhibitors. Archiv der Pharmazie, 350(12), 1700240. https://doi.org/10.1002/ardp.201700240 DOI: https://doi.org/10.1002/ardp.201700240
Eşkazan, E., Aker, R., Onat, F., Köseoğlu, S., Gören, M. Z., & Hasanoğlu, A. (1999). Effect of pirenzepine, a muscarinic M1 receptor antagonist, on amygdala kindling in rat. Epilepsy research, 37(2), 133-140. https://doi.org/10.1016/S0920-1211(99)00040-6 DOI: https://doi.org/10.1016/S0920-1211(99)00040-6
Español, A. J., & Sales, M. E. (2004). Different muscarinc receptors are involved in the proliferation of murine mammary adenocarcinoma cell lines. International journal of molecular medicine, 13(2), 311-317. DOI: https://doi.org/10.3892/ijmm.13.2.311
Feurino, L. W., Zhang, Y., Bharadwaj, U., Zhang, R., Li, F., Fisher, W. E., & Li, M. (2007). IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer biology & therapy, 6(7), 1096-1100. https://doi.org/10.4161/cbt.6.7.4328 DOI: https://doi.org/10.4161/cbt.6.7.4328
Figueroa-Valverde, L., Diaz-Cedillo, F., Lopez-Ramos, M., Garcia-Cervera, E., Pool-Gomez, E., Cardena-Arredondo, C., & Ancona-Leon, G. (2012). Glibenclamide-pregnenolone derivative has greater hypoglycemic effects and biodistribution than glibenclamide-OH in alloxan-rats. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 156(2), 122-127. DOI: https://doi.org/10.5507/bp.2012.028
Figueroa-Valverde, L., Rosas-Nexticapa, M., Alvarez-Ramirez, M., Lopez-Ramos, M., & Mateu-Armand, V. (2022). Theoretical evaluation of interaction of some dibenzo derivatives on both androgen receptor and 5α-reductase enzyme. Clinical Cancer Investigation Journal, 11(5-2022), 11-16. DOI: https://doi.org/10.51847/fIVMfELA7I
Fiszman, G. L., Middonno, M. C., de la Torre, E., Farina, M., Español, A. J., & Sales, M. E. (2007). Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer biology & therapy, 6(7), 1106-1113. https://doi.org/10.4161/cbt.6.7.4330 DOI: https://doi.org/10.4161/cbt.6.7.4330
Gentry P, Kokubo M, Bridges T. (2014). Discovery, Synthesis and Characterization of a Highly Muscarinic Acetylcholine Receptor (mAChR)‐Selective M5‐Orthosteric Antagonist, VU0488130 (ML381): A Novel Molecular Probe. ChemMedChem, 9(8): 1677-1682. https://doi.org/10.1002/cmdc.201402051
Gentry, P. R., Kokubo, M., Bridges, T. M., Cho, H. P., Smith, E., Chase, P., ... & Lindsley, C. W. (2014). Discovery, Synthesis and Characterization of a Highly Muscarinic Acetylcholine Receptor (mAChR)‐Selective M5‐Orthosteric Antagonist, VU0488130 (ML381): A Novel Molecular Probe. ChemMedChem, 9(8), 1677-1682. https://doi.org/10.1002/cmdc.201402051 DOI: https://doi.org/10.1002/cmdc.201402051
Haab, F., Stewart, L., & Dwyer, P. (2004). Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. European urology, 45(4), 420-429. DOI: https://doi.org/10.1016/j.eururo.2004.01.008
Halgren, T. A. (1996). Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Journal of computational chemistry, 17(5‐6), 490-519. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P
Hazan, S., Tauber, M., & Ben-Chaim, Y. (2024). Voltage dependence of M2 muscarinic receptor antagonists and allosteric modulators. Biochemical Pharmacology, 227, 116421. https://doi.org/10.1016/j.bcp.2024.116421 DOI: https://doi.org/10.1016/j.bcp.2024.116421
Holzgrabe U, Heller E. (2003) A new synthetic route to compounds of the AFDX-type with affinity to muscarinic M2-receptor. Tetrahedron, 59(6): 781-787. https://doi.org/10.1016/S0040-4020(02)01623-X DOI: https://doi.org/10.1016/S0040-4020(02)01623-X
Hoshi N, Ehlert F. (2024). Muscarinic Antagonists and Their Clinical Uses. Brody's Human Pharmacology-E-Book: Brody's Human Pharmacology-E-Book, 79.
Houghton, L. A., Rogers, J., Whorwell, P. J., Campbell, F. C., Williams, N. S., & Goka, J. (1997). Zamifenacin (UK‐76, 654), a potent gut M3 selective muscarinic antagonist, reduces colonic motor activity in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics, 11(3), 561-568. https://doi.org/10.1046/j.1365-2036.1997.00189.x DOI: https://doi.org/10.1046/j.1365-2036.1997.00189.x
Hwang, J. E., Lee, J. H., Park, M. R., Kim, D. E., Bae, W. K., Shim, H. J., & Chung, I. J. (2013). Blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in gastric cancer cells. Yonsei Medical Journal, 54(2), 374-380. https://doi.org/10.3349/ymj.2013.54.2.374 DOI: https://doi.org/10.3349/ymj.2013.54.2.374
Klasa-Mazurkiewicz, D., Jarząb, M., Milczek, T., Lipińska, B., & Emerich, J. (2011). Clinical significance of VEGFR-2 and VEGFR-3 expression in ovarian cancer patients. Polish Journal of Pathology, 62(1), 31-40.
Kohn, E. C., Alessandro, R., Probst, J., Jacobs, W., Brilley, E., & Felder, C. C. (1996). Identification and molecular characterization of a m5 muscarinic receptor in A2058 human melanoma cells: Coupling to inhibition of adenylyl cyclase and stimulation of phospholipase A2. Journal of Biological Chemistry, 271(29), 17476-17484. https://doi.org/10.1074/jbc.271.29.17476. DOI: https://doi.org/10.1074/jbc.271.29.17476
Kopparapu, P. K., Boorjian, S. A., Robinson, B. D., Downes, M., Gudas, L. J., Mongan, N. P., & Persson, J. L. (2013). Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer research, 33(6), 2381-2390.
Kwon, M. J., Kang, H. S., Choi, H. G., Kim, J. H., Kim, J. H., Bang, W. J., & Lee, H. K. (2023). Risk for esophageal cancer based on lifestyle factors–smoking, alcohol consumption, and body mass index: insight from a South Korean population study in a low-incidence area. Journal of Clinical Medicine, 12(22), 7086. https://www.mdpi.com/2077- 0383/12/22/7086# DOI: https://doi.org/10.3390/jcm12227086
Levitt, D. G. (2002). PKQuest: a general physiologically based pharmacokinetic model. Introduction and application to propranolol. BMC clinical pharmacology, 2, 1-21. DOI: https://doi.org/10.1186/1472-6904-2-5
Li, M., Huang, C., Wu, X., Ding, F., Hu, Z., Zhu, Y., & Tang, D. (2020). The optimization of a novel selective antagonist for human M2 muscarinic acetylcholine receptor. Bioorganic & Medicinal Chemistry Letters, 30(24), 127632. https://doi.org/10.1016/j.bmcl.2020.127632 DOI: https://doi.org/10.1016/j.bmcl.2020.127632
Liu, H., Xia, J., Wang, T., Li, W., Song, Y., & Tan, G. (2019). Differentiation of human glioblastoma U87 cells into cholinergic neuron. Neuroscience Letters, 704, 1-7. https://doi.org/10.1016/j.neulet.2019.03.049. DOI: https://doi.org/10.1016/j.neulet.2019.03.049
Liu, Y., Lu, L., Yang, H., Wu, X., Luo, X., Shen, J., & Li, M. (2023). Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: a review. Environmental Pollution, 339, 122730. https://doi.org/10.1016/j.envpol.2023.122730 DOI: https://doi.org/10.1016/j.envpol.2023.122730
Liu, Z., Zhang, Y., Lagergren, J., Li, S., Li, J., Zhou, Z., & Xie, S. H. (2023). Circulating sex hormone levels and risk of gastrointestinal cancer: systematic review and meta-analysis of prospective studies. Cancer Epidemiology, Biomarkers & Prevention, 32(7), 936-946. https://doi.org/10.1158/1055-9965.EPI-23-00396 DOI: https://doi.org/10.1158/1055-9965.EPI-23-0039
Lombardi, M. G., Negroni, M. P., Pelegrina, L. T., Castro, M. E., Fiszman, G. L., Azar, M. E., & Sales, M. E. (2013). Autoantibodies against muscarinic receptors in breast cancer: their role in tumor angiogenesis. PloS one, 8(2), e57572. https://doi.org/10.1371/journal.pone.0057572 DOI: https://doi.org/10.1371/journal.pone.0057572
Morris, G. M., Goodsell, D. S., Halliday, R. S., Huey, R., Hart, W. E., Belew, R. K., & Olson, A. J. (1998). Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of computational chemistry, 19(14), 1639-1662. DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Movahedi, N., Zendehdel, M., Vazir, B., & Hassanpour, S. (2024). Sericin-induced hypophagia mediates via M1 muscarinic, NMDA glutamate and glycine receptors in neonatal chicken. Archives of Razi Institute, 79(5), 1109-1116. https://doi.org/10.32592/ARI.2024.79.5.1109 DOI: https://doi.org/10.32592/ARI.2024.79.5.1109
Myslivecek J, Farar V. (2024). Autoradiography Assessment of Muscarinic Receptors in the Central Nervous System with a Special Focus on Specific the Selectivity Protocol for M1 M2. Muscarinic Receptor: From Structure to Animal Models. New York, NY: Springer US. 213-227. DOI: https://doi.org/10.1007/978-1-0716-4015-9_10
Nagano, H., Tomida, C., Yamagishi, N., & Teshima-Kondo, S. (2019). VEGFR-1 regulates EGF-R to promote proliferation in colon cancer cells. International Journal of Molecular Sciences, 20(22), 1-22. https://doi.org/10.3390/ijms20225608 DOI: https://doi.org/10.3390/ijms20225608
Nakajima, Y., Tsujimura, T., Tsutsui, Y., Chotirungsan, T., Kawada, S., Dewa, N., & Inoue, M. (2023). Atropine facilitates water-evoked swallows via central muscarinic receptors in anesthetized rats. American Journal of Physiology-Gastrointestinal and Liver Physiology, 325(2), G109-G121. DOI: https://doi.org/10.1152/ajpgi.00039.2023
Olianas, M. C., & Onali, P. (1999). PD 102807, a novel muscarinic M4 receptor antagonist, discriminates between striatal and cortical muscarinic receptors coupled to cyclic AMP. Life sciences, 65(21), 2233-2240. https://doi.org/10.1016/S0024-3205(99)00488-9 DOI: https://doi.org/10.1016/S0024-3205(99)00488-9
Pauling P, Petcher T. (1970). Interaction of atropine with the muscarinic receptor. Nature, 228(5272): 673-674. DOI: https://doi.org/10.1038/228673a0
Poland, A., & Glover, E. (1973). Studies on the mechanism of toxicity of the chlorinated dibenzo-p-dioxins. Environmental health perspectives, 5, 245-251. DOI: https://doi.org/10.1289/ehp.7305245
Rimmaudo, L. E., de la Torre, E., de Lustig, E. S., & Sales, M. E. (2005). Muscarinic receptors are involved in LMM3 tumor cells proliferation and angiogenesis. Biochemical and biophysical research communications, 334(4), 1359-1364. https://doi.org/10.1016/j.bbrc.2005.07.031 DOI: https://doi.org/10.1016/j.bbrc.2005.07.031
Rössler, J., Monnet, Y., Farace, F., Opolon, P., Daudigeos‐Dubus, E., Bourredjem, A., & Geoerger, B. (2011). The selective VEGFR1‐3 inhibitor axitinib (AG‐013736) shows antitumor activity in human neuroblastoma xenografts. International journal of cancer, 128(11), 2748-2758. https://doi.org/10.1002/ijc.25611 DOI: https://doi.org/10.1002/ijc.25611
Salem, A, Pulido P., Sanchez F et al. Effect of low dose metronomic therapy on MCF-7 tumor cells growth and angiogenesis. Role of muscarinic acetylcholine receptors. Int Immunopharm 2020; 84, 106514. https://doi.org/10.1016/j.intimp.2020.106514 DOI: https://doi.org/10.1016/j.intimp.2020.106514
Sertoglu, E., Yücel, Ç., Omma, A., Hayran, Y., Colak, S., Sandıkçı, S. C., & Ozgurtas, T. (2022). Determination of serum vascular endothelial growth factor (VEGF) and VEGF receptor levels with VEGF gene polymorphisms in patients with Behçet’s uveitis. Advances in Clinical and Experimental Medicine, 31(3), 231-240. https://doi.org/10.17219/acem/143586 DOI: https://doi.org/10.17219/acem/143586
Shah, N., Khurana, S., Cheng, K., & Raufman, J. P. (2009). Muscarinic receptors and ligands in cancer. American Journal of Physiology-Cell Physiology, 296(2), C221-C232. https://doi.org/10.1152/ajpcell.00514.2008 DOI: https://doi.org/10.1152/ajpcell.00514.2008
Shang, H. X., Ning, W. T., Sun, J. F., Guo, N., Guo, X., Zhang, J. N., & Wu, S. H. (2024). Investigation of the quality of life, mental status in patients with gynecological cancer and its influencing factors. World Journal of Psychiatry, 14(7), 1053. https://doi.org/10.5498%2Fwjp.v14.i7.1053 DOI: https://doi.org/10.5498/wjp.v14.i7.1053
Sicak, Y. (2021). Design and antiproliferative and antioxidant activities of furan-based thiosemicarbazides and 1, 2, 4-triazoles: their structure-activity relationship and SwissADME predictions. Medicinal Chemistry Research, 30(8), 1557-1568. DOI: https://doi.org/10.1007/s00044-021-02756-z
Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: Cancer Journal for Clinicians, 73(1), 17-48. https://doi.org/10.3322/caac.21820 DOI: https://doi.org/10.3322/caac.21763
Sloan, B., & Scheinfeld, N. S. (2008). Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Current opinion in investigational drugs (London, England: 2000), 9(12), 1324-1335.
Solis, F. J., & Wets, R. J. B. (1981). Minimization by random search techniques. Mathematics of operations research, 6(1), 19-30. DOI: https://doi.org/10.1287/moor.6.1.19
Tolaymat, M., Larabee, S. M., Hu, S., Xie, G., & Raufman, J. P. The Role of M3 Muscarinic Receptor Ligand-Induced Kinase Signaling in Colon Cancer Progression. Cancers (Basel). 2019; 11 (3). https://doi.org/10.3390/cancers11030308 DOI: https://doi.org/10.3390/cancers11030308
Tompkins, E., Mimic, B., Penn, R. B., & Pera, T. (2023). The biased M3 mAChR ligand PD 102807 mediates qualitatively distinct signaling to regulate airway smooth muscle phenotype. Journal of Biological Chemistry, 299(10), 1-12. DOI: https://doi.org/10.1016/j.jbc.2023.105209
Wang, J., Krysiak, P. S., Laurier, L. G., Sims, S. M., & Preiksaitis, H. G. (2000). Human esophageal smooth muscle cells express muscarinic receptor subtypes M1 through M5. American Journal of Physiology-Gastrointestinal and Liver Physiology, 279(5), G1059-G1069. https://doi.org/10.1152/ajpgi.2000.279.5.G1059. DOI: https://doi.org/10.1152/ajpgi.2000.279.5.G1059
Wang, Z., Wang, N., Han, S., Wang, D., Mo, S., Yu, L., ... & Chen, J. (2013). Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PloS one, 8(7), e68566. https://doi.org/10.1371/journal.pone.0068566 DOI: https://doi.org/10.1371/journal.pone.0068566
Wilhelm, S. M., Dumas, J., Adnane, L., Lynch, M., Carter, C. A., Schütz, G., ... & Zopf, D. (2011). Regorafenib (BAY 73‐4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International journal of cancer, 129(1), 245-255. https://doi.org/10.1002/ijc.25864 DOI: https://doi.org/10.1002/ijc.25864
Yakes, F. M., Chen, J., Tan, J., Yamaguchi, K., Shi, Y., Yu, P., & Joly, A. H. (2011). Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Molecular cancer therapeutics, 10(12), 2298-2308. https://doi.org/10.1158/1535-7163.MCT-11-0264 DOI: https://doi.org/10.1158/1535-7163.MCT-11-0264
Yu, H., Xia, H., Tang, Q., Xu, H., Wei, G., Chen, Y., & Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci Rep., 2017, 7, 40802. DOI: https://doi.org/10.1038/srep40802
Zhao, Q., Yue, J., Zhang, C., Gu, X., Chen, H., & Xu, L. (2015). Inactivation of M2 AChR/NF-κB signaling axis reverses epithelial-mesenchymal transition (EMT) and suppresses migration and invasion in non-small cell lung cancer (NSCLC). Oncotarget, 6(30), 29335. https://doi.org/10.18632/oncotarget.5004 DOI: https://doi.org/10.18632/oncotarget.5004
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Lauro Figueroa-Valverde, Marcela Rosas-Nexticapa, Magdalena Alvarez-Ramirez, Maria Virginia Mateu-Armad, Regina Cauich-Carrillo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


