Enhanced degradation of methylene blue dye using hydrothermally synthesized Nickel-doped Strontium oxide catalysts
DOI:
https://doi.org/10.14295/bjs.v3i12.663Keywords:
degradation, methylene blue, photocatalysis, wastewater treatment, hydrothermal synthesisAbstract
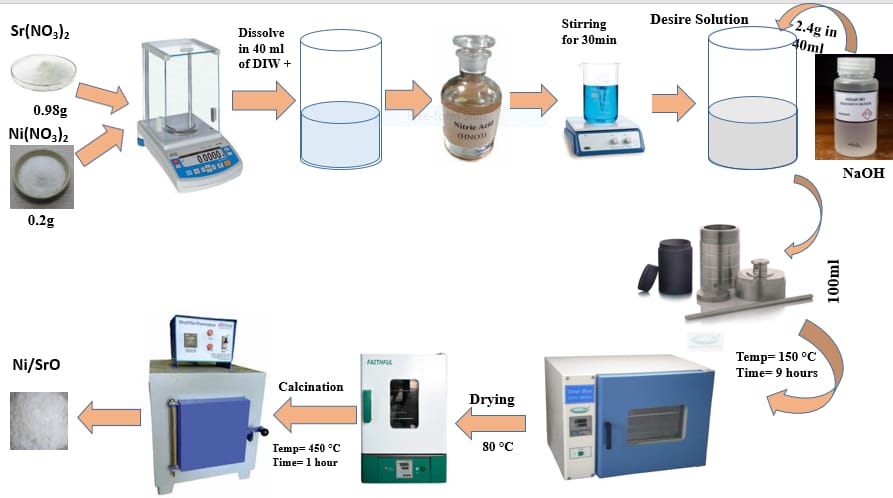
Methylene blue is an organic contaminant that is produced by the plastic, textile, and dye industries. Many studies have been undertaken to investigate the cleanup of methylene blue from industrial effluents. SrO nanoparticles are now being utilized to remove methylene blue colours from water. We used a hydrothermal technique to create strontium oxide nanoparticles for photocatalytic MB breakdown under light conditions. To enhance the solar light activity and avoid charge recombination, we employed a hydrothermal technique to add Ni as a dopant in strontium oxide nanoparticles. Strong base NaOH, nickel nitrate, and strontium nitrate were used as precursors. The nanoparticles were crushed into powder and calcined at 450 °C in a muffle furnace to produce SrO and Ni-doped SrO nanoparticles. The nanoparticles were analyzed using several analytical methods to determine their morphological and structural properties. At 309, 312, and 317 nm, UV-Vis spectroscopy showed absorbance values of SrO doped with varied nickel concentrations. The Ni–O stretching peak was identified in the FTIR analysis of strontium oxide nanoparticles at 402 cm-1 and 581 cm-1, whereas the Sr–O bond gave a signal at 854.84 cm-1. SEM images of Ni-doped SrO nanoparticles were created at various magnifications. The nanostrips are hexagonal and cylindrical. Sherrer's equation was used to compute the average crystalline structure, which showed that the diameters of pure and Ni-doped SrO (2 percent, 3 percent, and 4 percent) nanoparticles were 45.54 nm, 36.14 nm, 42.93 nm, and 41.21 nm, respectively. According to the EDX examination, the relative concentration of Ni-doped SrO is about 72 percent Sr and oxygen, with around 1.34 percent Ni. The resulting sample was tested for photocatalytic degradation of organic pollutants in aqueous solution, such as methylene blue, and the completion of the reaction was monitored using UV-visible spectroscopy to measure the % photocatalytic degradation during light illumination. According to the UV-visible spectra, 90% of the dye was effectively destroyed.
References
Ahsan, M., Qasim, S., Shah, A., Nawaz, I., Kashif, M., & Ahmad, W. J. B. J. O. S. (2024). Loading of anticancer drug anastrozole using Fe3O4@ SiO2. Brazilian Journal of Science, 3(2), 93-101. https://doi.org/10.14295/bjs.v3i2.497 DOI: https://doi.org/10.14295/bjs.v3i2.497
Akbari, B., Tavandashti, M. P., Zandrahimi, M. J. I. J. o. M. S., & Engineering. (2011). Particle size characterization of nanoparticles–a practical approach. Iranian Journal of Materials Science & Engineering, 8(2), 48-56. https://inis.iaea.org/search/search.aspx?orig_q=RN:43049756
Ali, N., Zada, A., Zahid, M., Ismail, A., Rafiq, M., Riaz, A., & Khan, A. J. J. o. t. C. C. S. (2019). Enhanced photodegradation of methylene blue with alkaline and transition‐metal ferrite nanophotocatalysts under direct sun light irradiation. Journal of the Chinese Chemical Society, 66(4), 402-408. https://doi.org/10.1002/jccs.201800213 DOI: https://doi.org/10.1002/jccs.201800213
Ali, S., Li, Z., Chen, S., Zada, A., Khan, I., Khan, I., Ali, W., Shaheen, S., Qu, Y., & Jing, L. (2019). Synthesis of activated carbon-supported TiO2-based nano-photocatalysts with well recycling for efficiently degrading high-concentration pollutants. Catalysis Today, 335, 557-564. https://doi.org/10.1016/j.cattod.2019.03.044 DOI: https://doi.org/10.1016/j.cattod.2019.03.044
Amin, M. T., Alazba, A. A., Manzoor, U. (2014). A review of removal of pollutants from water/wastewater using different types of nanomaterials. Advances in Materials Science and Engineering, (1), 825910. https://doi.org/10.1155/2014/825910 DOI: https://doi.org/10.1155/2014/825910
Athar, T. J. M. F. (2013). Synthesis and characterization of strontium oxide nanoparticles via wet process. Materials Focus, 2(6), 450-453. https://www.ingentaconnect.com/contentone/asp/mf/2013/00000002/00000006/art00004 DOI: https://doi.org/10.1166/mat.2013.1121
Budipramana, Y., Ersam, T., & Kurniawan, F. (2014). Synthesis nickel hydroxide by electrolysis at high voltage. ARPN Journal of Engineering and Applied Sciences, 9(11), 2074-2077. http://www.scopus.com/inward/record.url?scp=84911975219&partnerID=8YFLogxK
Davydov, A. A., & Sheppard, N. (2003). Molecular spectroscopy of oxide catalyst surfaces (Vol. 690): Wiley Chichester. https://doi.org/10.1002/0470867981 DOI: https://doi.org/10.1002/0470867981
Ghani, I., Kashif, M., Khattak, O. A., Shah, M., Nawaz, S., Ullah, S., Murad, S., Naz, S., Khan, H. W., Muhammad, S. & Jamal, M. (2023). Hydrothermal synthesis and characterization of Cobalt doped Bismuth oxide NPs for photocatalytic degradation of methyl orange dye. Journal of Xi’an Shiyou University, Natural Science Edition, 19(7), 1195-1217. https://www.xisdxjxsu.asia/viewarticle.php?aid=2506
Gupta, S. M., & Tripathi, M. (2012). An overview of commonly used semiconductor nanoparticles in photocatalysis. High Energy Chemistry, 46, 1-9. https://doi.org/10.1134/S0018143912010134 DOI: https://doi.org/10.1134/S0018143912010134
Hall, J. B., Dobrovolskaia, M. A., Patri, A. K., & McNeil, S. E. (2007). Characterization of nanoparticles for therapeutics. Nanomedicine, 2(6), 789-803. https://doi.org/10.2217/17435889.2.6.789 DOI: https://doi.org/10.2217/17435889.2.6.789
Humayun, M., Zada, A., Li, Z., Xie, M., Zhang, X., Qu, Y., Raziq, F., & Jing, L. (2016). Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Applied Catalysis B: Environmental, 180, 219-226. https://doi.org/10.1016/j.apcatb.2015.06.035 DOI: https://doi.org/10.1016/j.apcatb.2015.06.035
Jamal, M., Nabi, G. A. K., Sun, H., Ullah, K., Khattak, O. A., Kashif, M., Khan, S., Alam, M., Hussain, S., Ullah, M., Aleena, S., Haq, H., Umar, S., Atif, M., Hussain, I., & Masood, A. (2024). Preparation of Manganese-Doped Bismuth Oxide for the Photocatalytic Degradation of Methylene Blue. Archives of Advanced Engineering Science, 1-8. https://doi.org/10.47852/bonviewAAES42023402 DOI: https://doi.org/10.47852/bonviewAAES42023402
Jing, L., Qin, X., Luan, Y., Qu, Y., & Xie, M. (2012). Synthesis of efficient TiO2-based photocatalysts by phosphate surface modification and the activity-enhanced mechanisms. Applied Surface Science, 258(8), 3340-3349. https://doi.org/10.1016/j.apsusc.2011.07.101 DOI: https://doi.org/10.1016/j.apsusc.2011.07.101
Kashif, M., Ali, M., Naz, S., Amir, J., Murad, S., Atif, M., Khattak, O. A., Ukkah, S., Aleena, S., Khan, N. & Khan, M. Y. (2024a). Formulation development and characterization of quercetin loaded poly caprolactone nanoparticles for tumors. Brazilian Journal of Science, 3(2), 82-92. https://doi.org/10.14295/bjs.v3i2.494 DOI: https://doi.org/10.14295/bjs.v3i2.494
Kashif, M., Jawad, M., Khan, A. A., Sun, H., Ullah, K., Fakayode, O., & Azizi, S. (2024b). Fe/Ti-codoped strontium oxide nanoparticles for enhanced photocatalytic degradation of methyl orange. Journal of Applied Research in Water and Wastewater, 11(1), 8-14. https://doi.org/10.22126/arww.2024.10753.1336
Kashif, M., Khan, A. A., Sun, H., Kamal, J., Shah, M. I. A., Hussain, S., Amir, J., Jamal, Y., & Ahmad, T. (2024c). Synthesis and characterization of Fe-doped CuO nanoparticles: Catalytic efficiency in crystal violet dye degradation and exploration of electrical properties. Brazilian Journal of Science, 3(8), 1-18. https://doi.org/10.14295/bjs.v3i8.601 DOI: https://doi.org/10.14295/bjs.v3i8.601
Kashif, M., Muhammad, S., Ali, A., Ali, K., Khan, S., Zahoor, S., & Hamza, M. J(2023). Bismuth oxide nanoparticle fabrication and characterization for photocatalytic bromophenol blue degradation. Journal of Xi’an Shiyou University, Natural Science Edition, 19(07), 521-544. https://www.xisdxjxsu.asia/viewarticle.php?aid=2457
Khan, M., Hayat, A., Mane, S. K. B., Li, T., Shaishta, N., Alei, D., Zhao, T. K., Ullah, A., Zada, A., Rehman, A., & Khan, W. U. (2020). Functionalized nano diamond composites for photocatalytic hydrogen evolution and effective pollutant degradation. International Journal of Hydrogen Energy, 45(53), 29070-29081. https://doi.org/10.1016/j.ijhydene.2020.07.274 DOI: https://doi.org/10.1016/j.ijhydene.2020.07.274
Kumar, A., & Dixit, C. K. (2017). Methods for characterization of nanoparticles. In Advances in nanomedicine for the delivery of therapeutic nucleic acids (pp. 43-58): Elsevier. https://doi.org/10.1016/B978-0-08-100557-6.00003-1 DOI: https://doi.org/10.1016/B978-0-08-100557-6.00003-1
Larquet, C., & Carenco, S. (2020). Metal oxysulfides: from bulk compounds to nanomaterials. Frontiers in Chemistry, 8, 179. https://doi.org/10.3389/fchem.2020.00179 DOI: https://doi.org/10.3389/fchem.2020.00179
Lee, H., Park, Y.-K., Kim, S.-J., Kim, B.-H., & Jung, S.-C. (2015). Titanium dioxide modification with cobalt oxide nanoparticles for photocatalysis. Journal of Industrial and Engineering Chemistry, 32, 259-263. https://doi.org/10.1016/j.jiec.2015.08.025 DOI: https://doi.org/10.1016/j.jiec.2015.08.025
Li, F., Wangyang, P., Zada, A., Humayun, M., Wang, B., & Qu, Y. (2016). Synthesis of hierarchical Mn2O3 microspheres for photocatalytic hydrogen production. Materials Research Bulletin, 84, 99-104. https://doi.org/10.1016/j.materresbull.2016.07.032 DOI: https://doi.org/10.1016/j.materresbull.2016.07.032
Liu, L., Zhang, X., Yang, L., Ren, L., Wang, D., & Ye, J. (2017). Metal nanoparticles induced photocatalysis. National Science Review, 4(5), 761-780. https://doi.org/10.1093/nsr/nwx019 DOI: https://doi.org/10.1093/nsr/nwx019
Madani, S. S., Habibi-Yangjeh, A., Asadzadeh-Khaneghah, S., Chand, H., Krishnan, V., & Zada, A. (2021). Integration of Bi4O5I2 nanoparticles with ZnO: impressive visible-light-induced systems for elimination of aqueous contaminants. Journal of the Taiwan Institute of Chemical Engineers, 119, 177-186. https://doi.org/10.1016/j.jtice.2021.01.020 DOI: https://doi.org/10.1016/j.jtice.2021.01.020
Muhammad, S., Ali, A., Shah, J., Hamza, M., Kashif, M., Khel, B. K. A., & Iqbal, A. (2023). Using Moringa oleifera stem extract for green synthesis, characterization, and anti-inflammatory activity of silver oxide nanoparticles. Natural and Applied Sciences International Journal (NASIJ), 4(1), 80-97. https://doi.org/10.47264/idea.nasij/4.1.6 DOI: https://doi.org/10.47264/idea.nasij/4.1.6
Muhammad, S., Ali, A., Zahoor, S., Xinghua, X., Shah, J., Hamza, M., . . . Iqbal, A. J. A. S. A. P. V. (2023). Synthesis of Silver oxide nanoparticles and its antimicrobial, anticancer, anti-inflammatory, wound healing, and immunomodulatory activities - A review. Acta Scientific Applied Physics, 3(7). https://www.actascientific.com/ASAP/ASAP-03-0140.php
Niaz, M., Abrar, H., Ashfaq, S., Khan, N., Awais, M., e Baseerat, N., Jadoon, R., Abrar, A., Kashif, M., & Ullah, K. (2024). Qualitative phytochemical analysis and in vitro antibacterial activity of Punica granatum. Phytopharmacology Research Journal, 3(1), 31-38. https://www.ojs.prjn.org/index.php/prjn/article/view/64
Oskam, G. (2006). Metal oxide nanoparticles: synthesis, characterization and application. Journal of Sol-Gel Science and Technology, 37, 161-164. https://doi.org/10.1007/s10971-005-6621-2 DOI: https://doi.org/10.1007/s10971-005-6621-2
Qi, K., Xing, X., Zada, A., Li, M., Wang, Q., Liu, S.-Y., Lin, H., & Wang, G. J. C. I. (2020). Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: experimental and DFT studies. Ceramics International, 46(2), 1494-1502. https://doi.org/10.1016/j.ceramint.2019.09.116 DOI: https://doi.org/10.1016/j.ceramint.2019.09.116
Rabeie, B., Mahmoodi, N. M., & Mahkam, M. (2022). Morphological diversity effect of graphene quantum dot/MIL88A (Fe) composites on dye and pharmaceuticals (tetracycline and doxycycline) removal. Journal of Environmental Chemical Engineering, 10(5), 108321. https://doi.org/10.1016/j.jece.2022.108321 DOI: https://doi.org/10.1016/j.jece.2022.108321
Raziq, F., Aligayev, A., Shen, H., Ali, S., Shah, R., Ali, S., Bakhtiar, S. H., Ali, A., Zarshad, N., Zada, A., Xia, X., Zu, X., Khan, M., Wu, X., & Kong, Q. (2022). Exceptional photocatalytic activities of rGO modified (B, N) Co‐doped WO3, coupled with CdSe QDs for one photon Z‐scheme system: a joint experimental and dft study. Advanced Science, 9(2), 2102530. https://doi.org/10.1002/advs.202102530 DOI: https://doi.org/10.1002/advs.202102530
Sakar, M., Balakumar, S., Saravanan, P., & Jaisankar, S. N. (2013). Annealing temperature mediated physical properties of bismuth ferrite (BiFeO3) nanostructures synthesized by a novel wet chemical method. Materials Research Bulletin, 48(8), 2878-2885. https://doi.org/10.1016/j.materresbull.2013.04.008 DOI: https://doi.org/10.1016/j.materresbull.2013.04.008
Savage, N., & Diallo, M. S. (2005). Nanomaterials and water purification: opportunities and challenges. Journal of Nanoparticle Research, 7, 331-342. https://doi.org/10.1007/s11051-005-7523-5 DOI: https://doi.org/10.1007/s11051-005-7523-5
Shah, M., Hameed, A., Kashif, M., Majeed, N., Muhammad, J., Shah, N., Rehan, T., Khan, A., Uddin, J., Khan, A., & Kashtoh, H. (2024). Advances in agar-based composites: a comprehensive review. Carbohydrate Polymers, 346, 122619. https://doi.org/10.1016/j.carbpol.2024.122619 DOI: https://doi.org/10.1016/j.carbpol.2024.122619
Worku, A., Ayele, D., & Habtu, N. (2020). Recent advances and future perspectives in engineering of bifunctional electrocatalysts for rechargeable zinc – air batteries. Materials Today Advances, 9, 100116. https://doi.org/10.1016/j.mtadv.2020.100116 DOI: https://doi.org/10.1016/j.mtadv.2020.100116
Xiong, X., Zhang, Y., Wang, L., & Tsang, D. C. W. (2022). Chapter 1 - Overview of hazardous waste treatment and stabilization/solidification technology, in Low Carbon Stabilization and Solidification of Hazardous Wastes, Elsevier, 1-14. https://doi.org/10.1016/B978-0-12-824004-5.00031-1 DOI: https://doi.org/10.1016/B978-0-12-824004-5.00031-1
Xu, B., Zada, A., Wang, G., Qu, Y. (2019). Boosting the visible-light photoactivities of BiVO4 nanoplates by Eu doping and coupling CeO x nanoparticles for CO2 reduction and organic oxidation. Sustainable Energy & Fuels, 3(12), 3363-3369. https://doi.org/10.1039/C9SE00409B DOI: https://doi.org/10.1039/C9SE00409B
Yadav, N., Chaudhary, L., Sakhare, P., Dongale, T., Patil, P., Sheikh, A. D. (2018). Impact of collected sunlight on ZnFe2O4 nanoparticles for photocatalytic application. Journal of Colloid and Interface Science, 527, 289-297. https://doi.org/10.1016/j.jcis.2018.05.051 DOI: https://doi.org/10.1016/j.jcis.2018.05.051
Yarahmadi, M., Maleki-Ghaleh, H., Mehr, M. E., Dargahi, Z., Rasouli, F., & Siadati, M. H. (2021). Synthesis and characterization of Sr-doped ZnO nanoparticles for photocatalytic applications. Journal of Alloys and Compounds, 853, 157000. https://doi.org/10.1016/j.jallcom.2020.157000 DOI: https://doi.org/10.1016/j.jallcom.2020.157000
Yasmeen, H., Zada, A., Li, W., Xu, M., & Liu, S. (2019). Suitable energy platform of Bi2WO6 significantly improves visible-light degradation activity of g-C3N4 for highly toxic diuron pollutant. Materials Science in Semiconductor Processing, 102, 104598. https://doi.org/10.1016/j.mssp.2019.104598 DOI: https://doi.org/10.1016/j.mssp.2019.104598
Zada, A., Humayun, M., Raziq, F., Zhang, X., Qu, Y., Bai, L., Quin, C., Jing, L., & Fu, H. (2016). Exceptional visible‐light‐driven cocatalyst‐free photocatalytic activity of g‐C3N4 by well designed nanocomposites with plasmonic Au and SnO2. Advanced Energy Materials, 6(21), 1601190. https://doi.org/10.1002/aenm.201601190 DOI: https://doi.org/10.1002/aenm.201601190
Zada, A., Khan, M., Khan, M. A., Khan, Q., Habibi-Yangjeh, A., Dang, A., & Maqbool, M. J. E. R. (2021). Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environmental Research, 195, 110742. https://doi.org/10.1016/j.envres.2021.110742 DOI: https://doi.org/10.1016/j.envres.2021.110742
Zada, A., Khan, M., Qureshi, M. N., Liu, S.-y., & Wang, R. (2020). Accelerating photocatalytic hydrogen production and pollutant degradation by functionalizing g-C3N4 with SnO2. Frontiers in Chemistry, 7, 941. https://doi.org/10.3389/fchem.2019.00941 DOI: https://doi.org/10.3389/fchem.2019.00941
Zada, A., Qu, Y., Ali, S., Sun, N., Lu, H., Yan, R., & Jing, L. (2018). Improved visible-light activities for degrading pollutants on TiO2/g-C3N4 nanocomposites by decorating SPR Au nanoparticles and 2, 4-dichlorophenol decomposition path. Journal of Hazardous Materials, 342, 715-723. https://doi.org/10.1016/j.jhazmat.2017.09.005 DOI: https://doi.org/10.1016/j.jhazmat.2017.09.005
Zaman, S., Kashif, M., Shah, M., Hameed, A., Majeed, N., Ismail, M., & Khan, N. (2024). Investigating the enhanced photocatalytic degradation of bromophenol blue using Ni/Zn co-doped Strontium oxide nanoparticles synthesized via hydrothermal method. Brazilian Journal of Science, 3(1), 102-114. https://doi.org/10.14295/bjs.v3i1.460 DOI: https://doi.org/10.14295/bjs.v3i1.460
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Jehangir Shah, Hao Sun, Zijun Qiao, Talha Sharif, Misbah Gul

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


