Interaction of some chalcone derivatives with calcium channels using a theoretical model
DOI:
https://doi.org/10.14295/bjs.v3i11.658Keywords:
chalcone, derivatives, heart failure, nifedipine, amlodipineAbstract
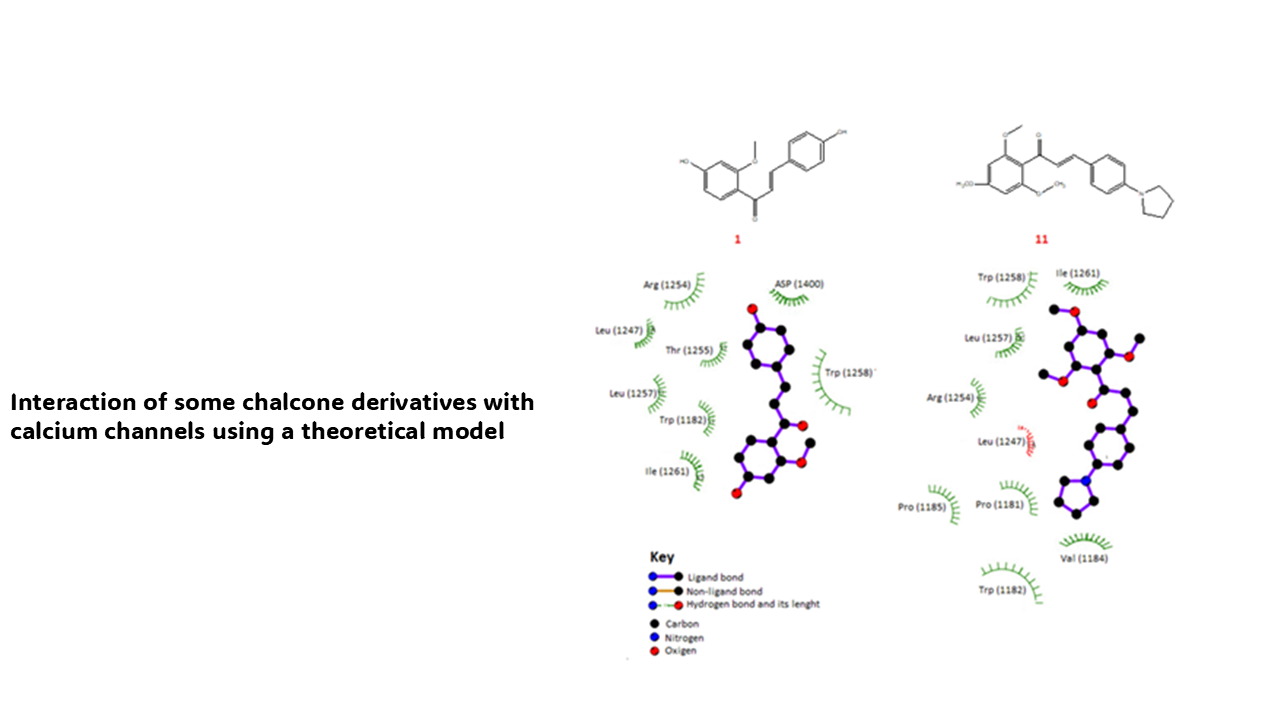
For several years, different drugs have been used to treat heart failure, such as digoxin, captopril, spironolactone, milrinone, levosimedam, dobutamine, and others. However, some of these drugs can produce secondary effects such as arrhythmia, cough, hyperkalemia, and others. Analyzing these data, this study aimed to evaluate the interaction of some chalcone derivatives (1-17) with calcium channels using theoretical models. It is important to mention that 7pjx protein, nifedipine, amlodipine, diltiazem, and verapamil were used as theoretical tools in the DockingServer program. The results showed differences in the interaction of chalcone derivatives compared with nifedipine, amlodipine, diltiazem, and verapamil drugs. Other data indicate that the inhibition constant (Ki) for chalcone analog 1 was lower compared with nifedipine, amlodipine, verapamil, and diltiazem. Besides, other results suggest that Ki for compound 11 was lower compared with nifedipine, verapamil, and diltiazem. All these data suggest that chalcone derivatives 1 and 11 could act as calcium channel inhibitors; this phenomenon could be translated into changes in blood pressure through a decrease in calcium intracellular levels. These data suggest that chalcone derivatives 1 and 11 could be good therapeutic alternatives to treat heart failure.
References
Akhtar, W., Butcher, C., Morley‐Smith, A., Riesgo-Gil, F., Dar, O., Baston, V., & Lyster, H. (2022). Oral milrinone for management of refractory right ventricular failure in patients with left ventricular assist devices. ESC Heart Failure, 9(6), 4340-4343. https://doi.org/10.1002/ehf2.14092 DOI: https://doi.org/10.1002/ehf2.14092
Alvarez-Ramirez, M., Figueroa-Valverde, L., Rosas-Nexticapa, M., Díaz-Cedillo, F., López-Ramos, M., & Hau-Heredia, L. (2024). Theoretical analysis of interaction between twenty-five cannabinoid derivatives with interleukin-6. Letters in Applied NanoBioscience. 13(3), 2024. https://doi.org/1.10 10.33263/LIANBS133.133 DOI: https://doi.org/10.14295/bjs.v3i7.573
Annapurna, A., Mudagal, M., & Ansari, A. (2012). Cardioprotective activity of chalcones in ischemia/reperfusion-induced myocardial infarction in albino rats. Experimental & Clinical Cardiology, 17(3), 110.
Anton, C., Cox, A., Watson, R., & Ferner, R. (2003). The safety of spironolactone treatment in patients with heart failure. Journal of Clinical Pharmacy and Therapeutics, 28(4), 285-287. https://doi.org/10.1046/j.1365-2710.2003.00491.x DOI: https://doi.org/10.1046/j.1365-2710.2003.00491.x
Arcidiacono, A., Cignoni, E., Mazzeo, P., Cupellini, L., & Mennucci, B. (2024). Predicting Solvatochromism of Chromophores in Proteins through QM/MM and Machine Learning. The Journal of Physical Chemistry A, 128(18), 3646-3658. https://doi.org/10.1021/acs.jpca.4c00249 DOI: https://doi.org/10.1021/acs.jpca.4c00249
Arif, R., Rana, M., Yasmeen, S. Khan, M. Abid, M. & Khan, M. S. (2020). Facile synthesis of chalcone derivatives as antibacterial agents: Synthesis, DNA binding, molecular docking, DFT and antioxidant studies. Journal of Molecular Structure, 1208, 127905. https://doi.org/10.1016/j.molstruc.2020.127905 DOI: https://doi.org/10.1016/j.molstruc.2020.127905
Aune, D., Schlesinger, S., Norat, T., & Riboli, E. (2019). Tobacco smoking and the risk of heart failure: A systematic review and meta-analysis of prospective studies. European journal of preventive cardiology, 26(3), 279-288. https://doi.org/10.1177/2047487318806658 DOI: https://doi.org/10.1177/2047487318806658
Bragazzi, N., Zhong, W., Shu, J., Abu-Much, A., Lotan, D., Grupper, A., & Dai, H. (2021). Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. European Journal of Preventive Cardiology, 28(15), 1682-1690. https://doi.org/10.1093/eurjpc/zwaa147 DOI: https://doi.org/10.1093/eurjpc/zwaa147
Chen, L., Tsai, M., Chern, C., Tsao, T., Lin, F., Chen, S., & Lin, C. S. (2020). A chalcone derivative, 1m‐6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation‐induced endothelial dysfunction. British Journal of Pharmacology, 177(23), 5375-5392. https://doi.org/10.1111/bph.15175 DOI: https://doi.org/10.1111/bph.15175
Cohn, J. & Tognoni, G. (2001). A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. New England Journal of Medicine, 345(23), 1667-1675. https://doi.org/10.1056/NEJMoa010713 DOI: https://doi.org/10.1056/NEJMoa010713
Di Muzio, E. Toti, D. & Polticelli, F. (2017). DockingApp: a user friendly interface for facilitated docking simulations with AutoDock Vina. Journal of Computer-Aided Molecular Design, 31, 213-218. DOI: https://doi.org/10.1007/s10822-016-0006-1
Dix, D. Houck, K. Martin, M. Richard, A. Setzer, R. & Kavlock, R. (2007). The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicological Sciences, 95(1), 5-12. https://doi.org/10.1093/toxsci/kfl103 DOI: https://doi.org/10.1093/toxsci/kfl103
Du, D., Yan, J., Ren, J., Lv, H., Li, Y., Xu, S., & Yu, S. (2013). Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. Journal of Medicinal Chemistry, 56(1), 97-108. https://doi.org/10.1021/jm301248y DOI: https://doi.org/10.1021/jm301248y
Eisenberg, M., Brox, A., & Bestawros, A. (2004). Calcium channel blockers: an update. The American Journal of Medicine, 116(1), 35-43. https://doi.org/10.1016/j.amjmed.2003.08.027 DOI: https://doi.org/10.1016/j.amjmed.2003.08.027
Fang, Q., Wang, J., Wang, L., Zhang, Y., Yin, H., Li, Y., & Zheng, C. (2015). Attenuation of inflammatory response by a novel chalcone protects kidney and heart from hyperglycemia-induced injuries in type 1 diabetic mice. Toxicology and Applied Pharmacology, 288(2), 179-191.https://doi.org/10.1016/j.taap.2015.07.009 DOI: https://doi.org/10.1016/j.taap.2015.07.009
Figueroa-Valverde, L., Díaz-Cedillo, F., Rosas-Nexticapa, M., Alvarez-Ramirez, M., Mateu-Armad, M., & López-Ramos, M. (2023). Interaction of some amino-nitrile derivatives with vascular endothelial growth factor receptor 1 (VEGFR1) using a theoretical model. Drug Research, 73(06), 355-364. DOI: https://doi.org/10.1055/a-2062-3571
Figueroa-Valverde, L., Marcela, R., Alvarez-Ramirez, M., Lopez-Ramos, M., Mateu-Armand, V., & Patricia, H. V. (2024). Interaction of thiophene and their derivatives with BRCA-1 using a theoretical model. Clinical Cancer Investigation Journal, 13(2-2024), 40-44. https://doi.org/10.51847/4AnibsrLIW DOI: https://doi.org/10.51847/4AnibsrLIW
Follmann, M., Ackerstaff, J., Redlich, G., Wunder, F., Lang, D., Kern, A., & Stasch, J. P. (2017). Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. Journal of Medicinal Chemistry, 60(12), 5146-5161.https://doi.org/10.1021/acs.jmedchem.7b00449 DOI: https://doi.org/10.1021/acs.jmedchem.7b00449
Granberg, K., Sakamaki, S., Larsson, N., Bergström, F., Fuchigami, R., Niwa, Y., & Lal, M. (2024). Discovery of clinical candidate AZD5462, a selective oral allosteric RXFP1 agonist for treatment of heart failure. Journal of Medicinal Chemistry, 67(6), 4419-4441. https://doi.org/10.1021/acs.jmedchem.3c02184 DOI: https://doi.org/10.1021/acs.jmedchem.3c02184
Guglin, M., Lynch, K., & Krischer, J. (2014). Heart failure as a risk factor for diabetes mellitus. Cardiology, 129(2), 84-92. https://doi.org/10.1159/000363282 DOI: https://doi.org/10.1159/000363282
Hernández, R., Genio, F., Casanova, J., Conato, M., & Paderes, M. (2024). Antiproliferative activities and SwissADME predictions of physicochemical properties of carbonyl group‐modified rotenone analogues. ChemistryOpen, 13(1), e202300087. https://doi.org/10.1002/open.202300087 DOI: https://doi.org/10.1002/open.202300087
Homans, S. (2007). Dynamics and thermodynamics of ligand–protein interactions. Bioactive Conformation I. 51-82. DOI: https://doi.org/10.1007/128_2006_090
Huang, C., Kohan, S., Liu, I., Lee, J., Baghdasaryan, N., Park, J., & Lee, M. (2024). Association between coronary artery disease testing in patients with new-onset heart failure and heart failure readmission and mortality. Journal of General Internal Medicine, 39(5), 747-755. DOI: https://doi.org/10.1007/s11606-023-08599-1
Huang, C., Park, J., Liu, I., Lee, J., Kohan, S., Mefford, M., & Lee, M. S. (2024). Effectiveness and safety of early treatment with spironolactone for new‐onset acute heart failure. Journal of Hospital Medicine, 19(4), 267-277. https://doi.org/10.1002/jhm.13317 DOI: https://doi.org/10.1002/jhm.13317
Ishaku, S., Bakare-Odunola, M., Musa, A., Yakasai, I., Garba, M., & Adzu, B. (2020). Effect of dihydro-artemisinin on the pharmacokinetics of gliclazide in diabetic subjects. International Journal of Biological and Chemical Sciences, 14(6), 2267-2276. https://doi.org/10.33263/BRIAC133.266 DOI: https://doi.org/10.4314/ijbcs.v14i6.27
Judson, R., Richard, A., Dix, D., Houck, K., Martin, M., Kavlock, R., & Smith, E. (2009). The toxicity data landscape for environmental chemicals. Environmental Health Perspectives, 117(5), 685-695. https://doi.org/10.1289/ehp.0800168 DOI: https://doi.org/10.1289/ehp.0800168
Kamimura, D., Cain, L., Mentz, R., White, W., Blaha, M., DeFilippis, A., & Hall, M. E. (2018). Cigarette smoking and incident heart failure: insights from the Jackson Heart Study. Circulation, 137(24), 2572-2582. https://doi.org/10.1161/CIRCULATIONAHA.117.031912 DOI: https://doi.org/10.1161/CIRCULATIONAHA.117.031912
Katsiki, N., Doumas, M., & Mikhailidis, D. (2016). Lipids, statins and heart failure: an update. Current Pharmaceutical Design, 22(31), 4796-4806. DOI: https://doi.org/10.2174/1381612822666160701073452
Khan, M., Shahid, I., Bennis, A., Rakisheva, A., Metra, M., & Butler, J. (2024). Global epidemiology of heart failure. Nature Reviews Cardiology, 1-18. DOI: https://doi.org/10.1038/s41569-024-01046-6
Lala, A., & Desai, A. S. (2014). The role of coronary artery disease in heart failure. Heart Failure Clinics, 10(2), 353-365. https://doi.org/10.1016/j.hfc.2013.10.002 DOI: https://doi.org/10.1016/j.hfc.2013.10.002
Lee, C., Lee, H. Yoon, M. Chun, K. Kong, M. Jung, M. & Kang, S. (2024). Heart failure statistics 2024 update: A report from the Korean society of heart failure. International Journal of Heart Failure, 6(2), 56. https://doi.org/10.36628%2Fijhf.2024.0010 DOI: https://doi.org/10.36628/ijhf.2024.0010
Levitt, D. (2002). PKQuest: a general physiologically based pharmacokinetic model. Introduction and application to propranolol. BMC Clinical Pharmacology, 2(1), 1-21. DOI: https://doi.org/10.1186/1472-6904-2-5
Li, J. Li, D., Xu, Y., Guo, Z., Liu, X., Yang, H., & Wang, L. (2017). Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents. Bioorganic & Medicinal Chemistry Letters, 27(3), 602-606. https://doi.org/10.1016/j.bmcl.2016.12.008 DOI: https://doi.org/10.1016/j.bmcl.2016.12.008
Lohachova, K., Sviatenko, A., Kyrychenko, A., Ivanov, V., Langer, T., Kovalenko, S., & Kalugin, O. (2024). Computer-aided drug design of novel nirmatrelvir analogs inhibiting main protease of Coronavirus SARS-CoV-2. Journal of Applied Pharmaceutical Science, 14(5), 232-239. https://doi.org/10.1080/07391102.2023.2192798 DOI: https://doi.org/10.7324/JAPS.2024.158114
Madani, A., Benkortbi, O., & Laidi, M. (2024). In silico prediction of the inhibition of new molecules on SARS-CoV-2 3CL protease by using QSAR: PSOSVR approach. Brazilian Journal of Chemical Engineering, 41(1), 427-442. DOI: https://doi.org/10.1007/s43153-023-00332-z
Maggioni, A., Latini, R., Carson, P., Singh, S., Barlera, S., Glazer, R., & Val-HeFT Investigators. (2005). Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). American Heart Journal, 149(3), 548-557. DOI: https://doi.org/10.1016/j.ahj.2004.09.033
Martin, S., Aday, A., Almarzooq, Z., Anderson, C., Arora, P., & Avery, C. (2024). 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation, 149(8), e347-e913. https://doi.org/10.1161/CIR.0000000000001209 DOI: https://doi.org/10.1161/CIR.0000000000001247
Masarone, D., Kittleson, M., Pollesello, P., Marini, M., Iacoviello, M., Oliva, F., & Pacileo, G. (2022). Use of levosimendan in patients with advanced heart failure: an update. Journal of Clinical Medicine, 11(21), 6408. https://www.mdpi.com/2077-0383/11/21/6408# DOI: https://doi.org/10.3390/jcm11216408
Mazimba, S., Jeukeng, C., Ondigi, O., Mwansa, H., Johnson, A., Elumogo, C., & Bilchick, K. (2023). Coronary perfusion pressure is associated with adverse outcomes in advanced heart failure. Perfusion, 38(7), 1492-1500. DOI: https://doi.org/10.1177/02676591221118693
Meng, W., Pi, Z., Brigance, R., Rossi, K., Schumacher, W., Bostwick, J., & Finlay, H. J. (2021). Identification of a hydroxypyrimidinone compound (21) as a potent APJ receptor agonist for the potential treatment of heart failure. Journal of Medicinal Chemistry, 64(24), 18102-18113. https://doi.org/10.1021/acs.jmedchem.1c01504 DOI: https://doi.org/10.1021/acs.jmedchem.1c01504
Nielsen, R., Pryds, K., Olesen, K., Mortensen, M., Gyldenkerne, C., Nielsen, J., & Maeng, M. (2024). Coronary artery disease is a stronger predictor of all‐cause mortality than left ventricular ejection fraction among patients with newly diagnosed heart failure: Insights from the WDHR. Journal of the American Heart Association, 13(14), e9771. https://doi.org/10.1161/JAHA.123.033938 DOI: https://doi.org/10.1161/JAHA.123.033938
Ohkuma, T., Komorita, Y., Peters, S., & Woodward, M. (2019). Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia, 62, 1550-1560. DOI: https://doi.org/10.1007/s00125-019-4926-x
Oliva, F., Comin-Colet, J., Fedele, F., Fruhwald, F., Gustafsson, F., Kivikko, M., & Tschöpe, C. (2018). Repetitive levosimendan treatment in the management of advanced heart failure. European Heart Journal Supplements, 20(suppl_I), I11-I20. https://doi.org/10.1093/eurheartj/suy040 DOI: https://doi.org/10.1093/eurheartj/suy040
Packer, M., Carver, J., Rodeheffer, R., Ivanhoe, R., DiBianco, R., & Zeldis, S. Promise Study Research Group*. (1991). Effect of oral milrinone on mortality in severe chronic heart failure. New England Journal of Medicine, 325(21), 1468-1475. https://doi.org/10.1056/NEJM19911121325210 DOI: https://doi.org/10.1056/NEJM199111213252103
Packer, M., Lee, W., Yushak, M., & Medina, N. (1986). Comparison of captopril and enalapril in patients with severe chronic heart failure. New England Journal of Medicine, 315(14), 847-853. DOI: https://doi.org/10.1056/NEJM198610023151402
Perozzo, R., Folkers, G., & Scapozza, L. (2004). Thermodynamics of protein–ligand interactions: history, presence, and future aspects. Journal of Receptors and Signal Transduction, 24(1-2), 1-52. https://doi.org/10.1081/RRS-120037896 DOI: https://doi.org/10.1081/RRS-120037896
Plewczynski, D., Philips, A., Grotthuss, M., Rychlewski, L., & Ginalski, K. (2014). HarmonyDOCK: the structural analysis of poses in protein-ligand docking. Journal of Computational Biology, 21(3), 247-256. https://doi.org/10.1089/cmb.2009.0111 DOI: https://doi.org/10.1089/cmb.2009.0111
Richter, J., Gunaga, P., Yadav, N., Bora, R., Bhide, R., Rajugowda, N., & Priestley, E. S. (2024). Discovery of BMS-986308: A renal outer medullary potassium channel inhibitor for the treatment of heart failure. Journal of Medicinal Chemistry, 67(11), 9731-9744. https://doi.org/10.1021/acs.jmedchem.4c00893 DOI: https://doi.org/10.1021/acs.jmedchem.4c00893
Riniker, S., Christ, C., Hansen, H., Hünenberger, P., Oostenbrink, C., Steiner, D., & van Gunsteren, W. (2011). Calculation of relative free energies for ligand-protein binding, solvation, and conformational transitions using the GROMOS software. The Journal of Physical Chemistry B, 115(46), 13570-13577. https://doi.org/10.1021/jp204303a DOI: https://doi.org/10.1021/jp204303a
Riswanto, F., Rawa, M., Murugaiyah, V., Salin, N., Istyastono, E., Hariono, M., & Wahab, H. A. (2021). Anti-cholinesterase activity of chalcone derivatives: synthesis, in vitro assay and molecular docking study. Medicinal Chemistry, 17(5), 442-452. https://doi.org/10.2174/1573406415666191206095032 DOI: https://doi.org/10.2174/1573406415666191206095032
Romankiewicz, J., Brogden, R., Heel, R., Speight, T., & Avery, G. (1983). Captopril: an update review of its pharmacological properties and therapeutic efficacy in congestive heart failure. Drugs, 25, 6-40. DOI: https://doi.org/10.2165/00003495-198325010-00002
Seidel, T., Bryant, S., Ibis, G., Poli, G., & Langer, T. (2017). 3D Pharmacophore modeling techniques in computer‐aided molecular design using ligandscout. Tutorials in Chemoinformatics, 279-309. https://doi.org/10.1002/9781119161110.ch20 DOI: https://doi.org/10.1002/9781119161110.ch20
Sicak, Y. (2021). Design and antiproliferative and antioxidant activities of furan-based thiosemicarbazides and 1, 2, 4-triazoles: their structure-activity relationship and SwissADME predictions. Medicinal Chemistry Research, 30(8), 1557-156 DOI: https://doi.org/10.1007/s00044-021-02756-z
Slivnick, J., & Lampert, B. (2019). Hypertension and heart failure. Heart Failure Clinics, 15(4), 531-541. https://doi.org/10.1016/j.hfc.2019.06.007 DOI: https://doi.org/10.1016/j.hfc.2019.06.007
Toropov, A. Toropova, A. Raska, I. Leszczynska, D. & Leszczynski, J. (2014). Comprehension of drug toxicity: software and databases. Computers in biology and medicine, 45, 20-25. https://doi.org/10.1016/j.compbiomed.2013.11.013 DOI: https://doi.org/10.1016/j.compbiomed.2013.11.013
Trosset, J., & Scheraga, H. (1999). PRODOCK: software package for protein modeling and docking. Journal of Computational Chemistry, 20(4), 412-427. https://doi.org/10.1002/(SICI)1096-987X(199903)20:4%3C412: AID-JCC3%3E3.0.CO;2-N DOI: https://doi.org/10.1002/(SICI)1096-987X(199903)20:4<412::AID-JCC3>3.0.CO;2-N
Vasan, R., & Levy, D. (1996). The role of hypertension in the pathogenesis of heart failure: a clinical mechanistic overview. Archives of Internal Medicine, 156(16), 1789-1796. https://doi.org/10.1001/archinte.1996.00440150033003 DOI: https://doi.org/10.1001/archinte.156.16.1789
Velagaleti, R., Massaro, J., Vasan, R., Robins, S., Kannel, W., & Levy, D. (2009). Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation, 120(23), 2345-2351. https://doi.org/10.1161/CIRCULATIONAHA.109.830984 DOI: https://doi.org/10.1161/CIRCULATIONAHA.109.830984
Wang, J., Hussain, S., Maddu, N., & Li, H. (2024). Protective effects of trans-chalcone on myocardial ischemia and reperfusion challenge through targeting phosphoinositide 3-kinase/Akt-inflammosome interaction. Journal of Physiological Investigation, 67(3), 129-138. https://doi.org/10.4103/ejpi.EJPI-D-24-00006 DOI: https://doi.org/10.4103/ejpi.EJPI-D-24-00006
Wilhelmsen, L., Rosengren, A., Eriksson, H., & Lappas, G. (2001). Heart failure in the general population of men–morbidity, risk factors and prognosis. Journal of Internal Medicine, 249(3), 253-261. https://doi.org/10.1111/j.1365-2796.2001.00801.x DOI: https://doi.org/10.1111/j.1365-2796.2001.00801.x
Yogeswaran, V., Hidano, D., Diaz, A., Van-Spall, H., Mamas, M., Roth, G., & Cheng, R. (2024). Regional variations in heart failure: a global perspective. Heart, 110(1), 11-18. https://doi.org/10.1136/heartjnl-2022-321295 DOI: https://doi.org/10.1136/heartjnl-2022-321295
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Lauro Figueroa-Valverde, Marcela Rosas-Nexticapa, Magdalena Alvarez-Ramirez , Emilio Aguilar-Sanchez, Maria Virginia Mateu-Armad; Enrique Bonilla-Zavaleta

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


