Interaction of dihydrofuran-2-one and its derivatives with either MAO-B or COMT enzymes using a theoretical model
DOI:
https://doi.org/10.14295/bjs.v3i10.634Keywords:
Parkinson´s, MAO-B, COMT, dihydrofuran-2-one, derivative, tolcaponeAbstract
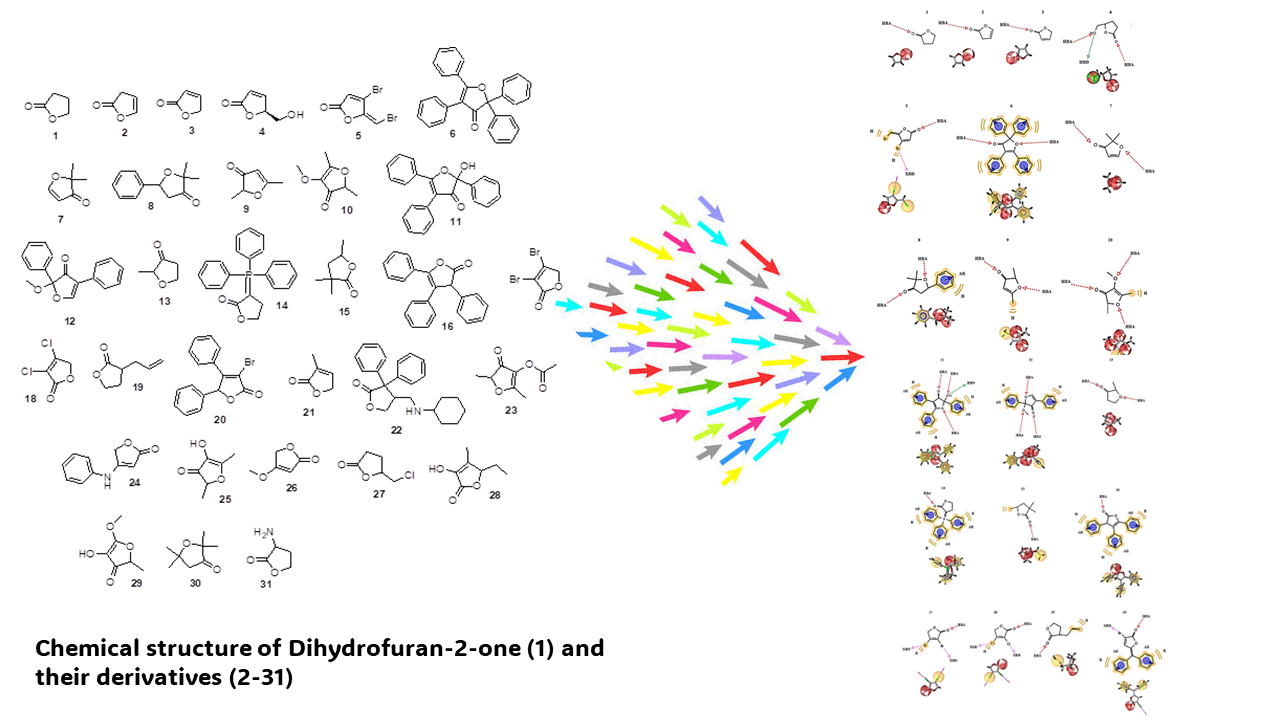
There are several drugs for treating Parkinson's such as L-Dopa, carbidopa, benserazide, entacapone, bromocriptine, safinamide, rasagiline, and others. However, some of these drugs can produce some secondary effects such as hypotension, insomnia, dizziness, nausea, and constipation. In the search for a new therapeutic alternative for treating Parkinson´s, this study aimed to evaluate the theoretical interaction of Dehydrofuran-2-one (1) and their derivatives (2-31) with both MAO-B and COMT enzymes. To evaluate the interaction of Dehydrofuran-2-one (1) and their derivatives (2-31) with both MAO-B and COMT enzymes, the 1gos and 1vid proteins as theoretical tools. Besides, some drugs, such as selegiline, rasagiline, safinamide, entacapone, and tolcapone, were used as controls in the DockingServer program. The results showed differences in the interaction of compounds 1-31 with either 1gos or 1vid proteins surface compared to the controls. Other data showed that inhibition constants (Ki) for 2, 3, 12, and 26 were lower compared to selegeline, rosagiline, and sofinamide, respectively. In addition, the Ki for 1-3, 7, 9, 10, 13, 21, and 25 were lower than entacapone and tolcapone. These data suggest that 1-3, 12, and 26 could act as MAO-B inhibitors and compounds 1-3, 7, 9, 10, 13, 21, and 26 as COMT antagonists. In conclusion, these compounds may be a good therapeutic alternative for treating Parkinson´s disease.
References
Abdel-Magid, A. (2019). LRRK2 kinase inhibitors as possible therapy for Parkinson’s disease and other neurodegenerative disorders. ACS Medicinal Chemistry Letters Journal, 10(6), 846-847. https://doi.org/10.1021/acsmedchemlett.9b00216
Alexander, G. (2004). Biology of Parkinson's disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues in Clinical Neuroscience, 6(3), 259-280. https://doi.org/10.31887/DCNS.2004.6.3/galexander
Bakchi, B., Krishna, A., Sreecharan, E., Ganesh, V., Niharika, M., & Maharshi, S. (2022). An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: A medicinal chemist's perspective. Journal of Molecular Structure, 1259, 132712. https://doi.org/10.1016/j.molstruc.2022.132712
Baskaran, K., Arumugam, A., Kandasamy, R., & Alagarsamy, S. (2020). In silico method for prediction of maximum binding affinity and ligand-protein interaction studies on Alzheimer’s disease. International Journal of Research - GRANTHAALAYAH, 8, 362-370. https://doi.org/10.29121/granthaalayah.v8.i11.2020.2472
Basu, S., Barawkar, D., Ramdas, V., Naykodi, M., Shejul, Y., & Patel, M. (2017). Discovery of potent and selective A2A antagonists with efficacy in animal models of Parkinson’s disease and depression. ACS Medicinal Chemistry Letters Journal, 8(8), 835-840. https://doi.org/10.1021/acsmedchemlett.7b00175
Billingsley, K., Bandres-Ciga, S., Saez-Atienzar, S., & Singleton, A. (2018). Genetic risk factors in Parkinson’s disease. Cell and Tissue Research, 373, 9-20.
Blesa, J., Foffani, G., Dehay, B., Bezard, E., & Obeso J. (2022). Motor and non-motor circuit disturbances in early
Parkinson disease: which happens first?. Nature Reviews Neuroscience, 23(2),115-28.
Borchardt, R. (1973). Catechol O-methyltransferase. 2. In vitro inhibition by substituted 8-hydroxyquinolines. Journal of Medicinal Chemistry, 16(4), 382-387. https://doi.org/10.1021/jm00262a016
Burkhard, P., Dominici, P., Borri-Voltattorni, C., Jansonius, J., & Malashkevich, V. (2001) Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nature Structural & Molecular Biology, 8(11), 963-967. https://doi.org/10.1038/nsb1101-963
Butina, D., Segall, M., & Frankcombe, K. (2002). Predicting ADME properties in silico: methods and models. Drug Discovery Today, 7(11), 83-88. https://doi.org/10.1016/S1359-6446(02)02288-2
Carotti, A., Carrieri, A., Chimichi, S., Boccalini, M., Cosimelli, B., & Gnerre, C. (2002). Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorganic & Medicinal Chemistry Letters, 12(24), 3551-3555. https://doi.org/10.1016/S0960-894X(02)00798-9
Choi, H. L., Ahn, J. H., Chang, W. H., Jung, W., Youn, J., & Shin, D. W. (2023). Risk of Parkinson’s Disease in Stroke Survivors: A nationwide cohort study in South Korea. MedRxiv, 2023-2026. https://doi.org/10.1101/2023.06.02.23290911
Churchyard, A., Mathias, C., Boonkongchuen, P., & Lees, A. (1997). Autonomic effects of selegiline: possible cardiovascular toxicity in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 63(2), 228-234. https://doi.org/10.1136/jnnp.63.2.228
Dezsi, L., & Vecsei, L. (2017). Monoamine oxidase B inhibitors in Parkinson's disease. Current Drugs Targets- CNS & Neurological Disorders, 16(4), 425-439. https://doi.org/10.2174/1871527316666170124165222
Dowell, F., & Lee, J. (1970). Levodopa, Parkinson's disease, and hypotension. Annals of Internal Medicine, 72(5), 751-2.
Dulski, J., Uitti, J., Ross, O., & Wszolek, Z. (2022). Genetic architecture of Parkinson’s disease subtypes-Review of the literature. Frontiers in Aging Neuroscience, 14, 1023574. https://doi.org/10.3389/fnagi.2022.1023574
Edmondson, D., & Binda, C. (2018). Monoamine oxidases. Membrane protein complexes. Structure and function, 117-39.
Ernst, G., Akuma, D., Au, V., Buchler, I., Byers, S., Carr, G., & Barrow, J. (2019). Synthesis and evaluation of bicyclic hydroxypyridones as inhibitors of catechol O-methyltransferase. ACS Medicinal Chemistry Letters Journal, 10(11), 1573-8. https://doi.org/10.1021/acsmedchemlett.9b00345
Fabbri, M., Ferreira, & J., Rascol, O. (2022). COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs, 36(3), 261-82.
Fabbri, M., Ferreira, J., & Rascol, O. (2022). COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs, 36(3), 261-82.
Fahn, S. (2018). The history of dopamine and levodopa in the treatment of Parkinson's disease. Movement disorders: Official Journal of the Movement Disorder Society, 23(3), 497-508. https://doi.org/10.1002/mds. 22028
Figueroa-Valverde, L., Diaz-Cedillo, F., Nexticapa, M., Alvarez-Ramirez, M., López-Ramos, M., & Melgarejo-Guttierrez, M. (2024). Biochemical interaction of twenty steroid derivatives with ribosomal protein kinase 4 S6 (RSK-4) surface using a theoretical model. Brazilian Journal of Science, 3(2), 66-81. https://doi.org/10.14295/bjs.v3i2.482
Gianutsos, G., Chute, S., & Dunn, J. (1995). Pharmacological changes in dopaminergic systems induced by long-term administration of amantadine. European Journal of Pharmacology, 110(3), 357-61. https://doi.org/10.1016/0014-2999(85)90564-3
Halgren, T. (1996). Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Journal of Computational Chemistry, 17(5‐6), 490-519. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P
Harrison, S., Poslusney, M., Mulhearn, J., Zhao, Z., Kett, N., & Schubert, J. (2015). Synthesis and evaluation of heterocyclic catechol mimics as inhibitors of catechol-O-methyltransferase (COMT). ACS Medicinal Chemistry Letters Journal, 6(3), 318-323. https://doi.org/10.1021/ml500502d
Kaltenboeck, A., Halahakoon, D., Harmer, C., Cowen, P., & Browning, M. (2022). Enhanced taste recognition following subacute treatment with the dopamine D2/D3 receptor agonist pramipexole in healthy volunteers. International Journal of Neuropsychopharmacology, 25(9), 720-726. https://doi.org/10.1093/ijnp/pyac030
Kang, M., An, J., Li, H., Zhuang, W., Heo, R., Park, S., & Park, W. (2022). Blockade of voltage-dependent K+ channels by benztropine, a muscarinic acetylcholine receptor inhibitor, in coronary arterial smooth muscle cells. Toxicological Sciences, 189(2), 260-267. https://doi.org/10.1093/toxsci/kfac083
Kasdagli, M., Katsouyanni, K., Dimakopoulou, K., & Samoli, E. (2019). Air pollution and Parkinson's disease: a systematic review and meta-analysis up to 2018. International Journal of Hygiene and Environmental Health, 222(3), 402-409. https://doi.org/10.1016/j.ijheh.2018.12.006
Khrapova, M., Khrapov, S., Chechushkov, A., Kozhin, P., Romakh, L., & Serykh, A. (2023). The toxicity of a new monophenolic synthetic inducer of keap1/Nrf2/ARE redox-sensitive signaling system in vitro and in vivo. Cell and Tissue Biology, 17(3), 299-305. https://doi.org/10.1134/S1990519X23030069
Kiss, I., Ferreira, H., Torrão, L., Bonifácio, M., Palma, P., & Soares, P. (2010). Discovery of a long-acting, peripherally selective inhibitor of Catechol-O-methyltransferase. Journal of Medicinal Chemistry, 53(8), 3396-3411. https://doi.org/10.1021/jm1001524
Learmonth, D., Palma, P., Vieira-Coelho, M., & Soares, P. (2004). Synthesis, biological evaluation, and molecular modeling studies of a novel, peripherally selective inhibitor of Catechol-O-methyltransferase. Journal of Medicinal Chemistry, 47(25), 6207-6217. https://doi.org/10.1021/jm040848o
Lee, D. (1993). MAO Inhibitors in Parkinson's disease. Journal of the Korean Neurological Association, 11(1), 1-7. 10.5607/en.2011.20.1.1
Lohmann, S., Grigoletto, J., Bernis, M., Pesch, V., Ma, L., & Reithofer, S. (2022). Ischemic stroke causes Parkinson’s disease-like pathology and symptoms in transgenic mice overexpressing alpha-synuclein. Acta Neuropathologica Communications, 10(1), 1-17.
Lopez-Ramos, M., Figueroa-Valverde, L., Rosas-Nexicapa, M., Cervantes-Ortega, C., Alvarez-Ramirez, M., & Diaz-Cedillo, F. (2024). Interaction of benzenesulfonamide derivatives with Smyd3 using a theoretical model. Brazilian Journal of Science, 3(1), 115-129. https://doi.org/10.14295/bjs.v3i1.455
Marconi, R., Bonnet, A., Vidailhet, M., & Agid, Y. (1992). The IMAO-B MDL 72.974 A in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 55(11), 1096. https://doi.org/10.1136/jnnp.55.11.1096-a
Martin, M., Dix, D., Judson, R., Kavlock, R., Reif, D., & Richard, A. (2010). Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA’s ToxCast program. Chemical Research in Toxicology Journal, 23(3), 578-590. https://doi.org/10.1021/tx900325g
Montioli, R., Voltattorni, S., & Bertoldi, M. (2016). Parkinson’s disease: recent updates in the identification of human dopa decarboxylase inhibitors. Current Drug Metabolism, 17(5), 513-518.
Morris, G., Huey, R., & Olson, A. (2008). Using autodock for ligand‐receptor docking. Current Protocols in Bioinformatics, 24(1), 8-14. https://doi.org/10.1002/0471250953.bi0814s24
Murata, H., Barnhill, L., & Bronstein, J. (2022). Air pollution and the risk of Parkinson's disease: a review. Movement Disorders, 37(5), 894-904. https://doi.org/10.1002/mds.28922
Niswender, C., Johnson, K., Weaver, C., Jones, C., Xiang, Z., & Luo, Q. (2008). Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Molecular Pharmacology, 74(5), 1345-1358. https://doi.org/10.1124/mol.108.049551
Olanow C. (2000). Tolcapone and hepatotoxic effects. Archives of Neurology, 57(2), 263-267. https://doi.org/10.1002/ 0471250953.bi0814s24
Pagano, G., Ferrara, N., Brooks, D., & Pavese, N. (2016). Age at onset and Parkinson disease phenotype. Neurology, 86(15), 1400-1407. https://doi.org/10.1212/WNL.00000000000024
Palacios, N. (2017). Air pollution and Parkinson’s disease-evidence and future directions. Reviews on Environmental Health, 32(4), 303-313. https://doi.org/10.1515/reveh-2017-0009
Panarese, J., Engers, D., Wu, Y., Bronson, J., Macor, J., Chun, A., & Lindsley, C. (2018). Discovery of VU2957 (Valiglurax): An mGlu4 positive allosteric modulator evaluated as a preclinical candidate for the treatment of Parkinson’s disease. ACS Medicinal Chemistry Letters Journal, 10(3), 255-260.
Park, H., Kim, J., Kim, T., Jo, S., Yeom, M., Moon, B., & Choo, H. (2013). Oxazolopyridines and thiazolopyridines as monoamine oxidase B inhibitors for the treatment of Parkinson’s diseas. Bioorganic & Medicinal Chemistry, 21(17), 5480-5487. https://doi.org/10.1016/j.bmc.2013.05.066
Patel, Y., Gillet, V., Bravi, G., & Leach, A. (2002). A comparison of the pharmacophore identification programs: Catalyst, DISCO and GASP. Journal of Computer-Aided Molecular Design, 16, 653-681.
Peeters, M., Maloteaux, J., & Hermans, E. (2003). Distinct effects of amantadine and memantine on dopaminergic transmission in the rat striatum. Neuroscience Letters, 343(3), 205-209. https://doi.org/10.1016/S0304-3940(03)00398-7
Pérez, S., La-Farré, M., Garcıá, M., & Barceló, D. (2001). Occurrence of polycyclic aromatic hydrocarbons in sewage sludge and their contribution to its toxicity in the ToxAlert® 100 bioassay. Chemosphere, 45(6-7), 705-712. https://doi.org/10.1016/S0045-6535(01)00152-7
Raeisi, S. (2013). Molecular docking approach of monoamine oxidase B inhibitors for identifying new potential drugs: Insights into drug-protein interaction discovery. Journal of Cell and Molecular Research, 5(1), 24-33.
Sashidhara, K., Modukuri, R., Jadiya, P., Rao, K., Sharma, T., & Haque, R. (2014). Discovery of 3-Arylcoumarin-tetracyclic tacrine hybrids as multifunctional agents against Parkinson’s disease. ACS Medicinal Chemistry Letters Journal, 5(10), 1099-1103. https://doi.org/10.1021/ml500222g
Scatton, B., Javoy-Agid, F., Rouquier, L., Dubois, B. & Agid, Y. (1983). Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Research, 275(2), 321-328.
Shen, Z., & Kong, D. (2018). Meta-analysis of the adverse events associated with extended-release versus standard immediate-release pramipexole in Parkinson disease. Medicine, 97(34), e11316. https://doi.org/10.1097/MD.0000000000011316
Shinde, V., Hoelting, L., Srinivasan, S., Meisig, J., Meganathan, K., & Jagtap, S. (2017). Definition of transcriptome-based indices for quantitative characterization of chemically disturbed stem cell development: introduction of the STOP-Tox ukn and STOP-Tox ukk tests. Archives of Toxicology, 91, 839-864.
Song, L., Zhang, S., Li, H., Hansson, O., Sonestedt, E., & Borné, Y. (2022). Comparison of risk factors for Parkinson’s disease, coronary events and ischemic stroke. npj Parkinson's Disease, 8(1), 107.
Visha, K., Singla, C., Sharma, A., & Dhiman, A. (2020). Prediction of Environmental Toxicity of Active Chemical Constituents of Ipomoea Carnea through GUSAR Software. Turkish Journal of Mathematics Education, 11(2), 735-740. https://doi.org/10.17762/turcomat.v11i2.9768
Wang, H., Mulgaonkar, N., Pérez, L., & Fernando, S. (2022). ELIXIR-A: An Interactive Visualization Tool for Multi-Target Pharmacophore Refinement. ACS Omega, 7(15), 12707-12715. https://doi.org/10.1021/acsomega.1c07144
Wang, Y., Deng, W., Lee, D., Yan, L., Lu, Y., Dong, S., & Jiang, W. (2024). Age-associated disparity in phagocytic clearance affects the efficacy of cancer nanotherapeutics. Nature Nanotechnology, 19(2), 255-263.
Wang, Z., Yi, C., Chen, K., Wang, T., Deng, K., & Jin, C. (2022). Enhancing monoamine oxidase B inhibitory activity via chiral fluorination: Structure-activity relationship, biological evaluation, and molecular docking study. European Journal of Medicinal Chemistry, 228, 114025. https://doi.org/10.1016/j.ejmech.2021.114025
Willis, A., Roberts, E., Beck, J., Fiske, B., Ross, W., & Savica R. (2022). Incidence of Parkinson disease in North America. Npj Parkinson's Disease, 8(1), 1-7. https://doi.org/10.1038/s41531-022-00410-y
Wolbe, G., & Langer, T. (2005). LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. Journal of Chemical Information and Modeling, 45(1), 160-169. https://doi.org/10.1016/S0960-894X(02)00798-9
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Lauro Figueroa-Valverde, Marcela Rosas-Nexticapa, Magdalena Alvarez-Ramirez, Emilio Aguilar-Sanchez, Maria Virginia Mateu-Armad

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


