Loading of anticancer drug anastrozole using Fe3O4@SiO2
DOI:
https://doi.org/10.14295/bjs.v3i2.497Keywords:
Fe3O4, Fe3O4@SiO2, anastrozole loading, adsorptionAbstract
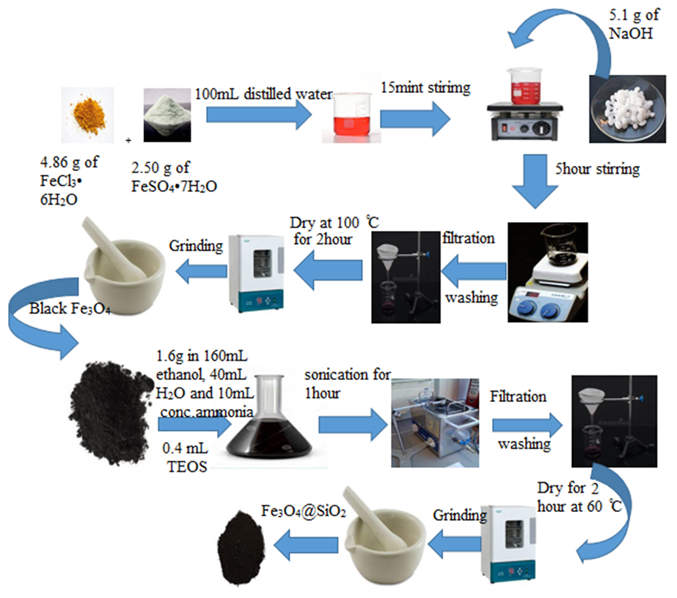
Anastrozole is a prescription drug that is used to treat hormone-dependent breast cancer, mostly in women who have gone through menopause. Once a day, it is taken by mouth. Anastrozole stops the activity of an enzyme called aromatase, which changes androgens into oestrogens. But taking the drug often comes with side effects that depend on how much you take, such as tiredness, diarrhea, hot flashes, nausea, headaches, muscle and joint pain, and so on. Anastrozole has also been linked to other side effects and more bone loss. To overcome the side effects of anastrozole and for their efficient delivery anastrozole must be loaded on the surfaces which is biocompatible and stable towards human body. So, the co-precipitation method was used to make iron oxide nanoparticles, which were then covered with silica using the Stober method. The made Fe3O4@SiO2 nanocomposite was taken out as a black powder and studied using FTIR, EDX, and SEM. The SEM picture showed that the Fe3O4 and Fe3O4@SiO2 nanoparticles size ranges were between 30 and 45 nm and 55 to 70 nm respectively. We also looked at how contact time, pH, and the amount of nanocomposite affected the loading of the drug. The best adsorption (85.6%) happened when the reaction lasted 12 h, the pH was 4, and the adsorbent dose was 10 mg.
References
Ahangaran, F., Hassanzadeh, A., & Nouri, S. (2013). Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. International Nano Latters, 3, 1-5. https://doi.org/10.1186/2228-5326-3-23 DOI: https://doi.org/10.1186/2228-5326-3-23
Asab, G., Zereffa, E. A., & Seghne, T. A. (2020). Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: Characterization and evaluation of antimicrobial activity. International Journal of Biomaterials, 2020, 1-11. https://doi.org/10.1155/2020/4783612 DOI: https://doi.org/10.1155/2020/4783612
Ghasemzadeh, M. A., Abdollahi-Basir, M. H., & Babaei, M. (2015). Fe3O4@SiO2–NH2 core-shell nanocomposite as an efficient and green catalyst for the multi-component synthesis of highly substituted chromeno [2, 3-b]pyridines in aqueous ethanol media. Green Chemistry Letters and Reviews, 8(3-4), 40-49. https://doi.org/10.1080/17518253.2015.1107139 DOI: https://doi.org/10.1080/17518253.2015.1107139
Gul, S., Shah, N., Arain, M. B., Rahman, N., Rehan, T., Ul-Islam, M., Ullah, M. W., & Yang, G. (2019). Fabrication of magnetic core shell particles coated with phenylalanine imprinted polymer. Polymer Testing, 75, 262-269. https://doi.org/10.1016/j.polymertesting.2019.02.023 DOI: https://doi.org/10.1016/j.polymertesting.2019.02.023
Hariani, P.L., Faizal, M. and Setiabudidaya, D. (2013). Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. International Journal of Environmental Science and Development, 4(3),336. DOI: https://doi.org/10.7763/IJESD.2013.V4.366
Homogen, M. (2018). Synthesis and physicochemical properties of magnetite nanoparticles (Fe3O4) as potential solid support for homogeneous catalysts. Malaysian Journal of Analytical Sciences, 22(5), 768-774. https://doi.org/10.17576/mjas-2018-2205-04 DOI: https://doi.org/10.17576/mjas-2018-2205-04
Hwang, S.W., Umar, A., Dar, G.N., Kim, S.H. and Badran, R.I. (2014). Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sensor Letters, 12(1), 97-101. https://doi.org/10.1166/sl.2014.3224 DOI: https://doi.org/10.1166/sl.2014.3224
Javidi, J., Esmaeilpour, M., & Khansari, M.R. (2015). Synthesis, characterization and application of core–shell magnetic molecularly imprinted polymers for selective recognition of clozapine from human serum. RSC Advances, 5(89), 73268-73278. https://doi.org/10.1039/C5RA10356H DOI: https://doi.org/10.1039/C5RA10356H
Kralj, S., Drofenik, M., & Makovec, D. (2011). Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups. Journal of Nanoparticle Research, 13,2829-2841. https://doi.org/10.1007/s11051-010-0171-4 DOI: https://doi.org/10.1007/s11051-010-0171-4
Li, L., Gao, F., Jiang, W., Wu, X., Cai, Y., Tang, J., Gao, X., & Gao, F. (2016). Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Delivery, 23(5), 726-1733. https://doi.org/10.3109/10717544.2015.1006404 DOI: https://doi.org/10.3109/10717544.2015.1006404
Liu, H., Wang, Q. and Zhang, F. (2020). Preparation of Fe3O4@SiO2@ P (AANa-co-AM) composites and their adsorption for Pb (II). ACS Omega, 5(15), 8816-8824. https://doi.org/10.1021/acsomega.0c00403 DOI: https://doi.org/10.1021/acsomega.0c00403
Sodipo, B. K., & Aziz, A. A. (2015). Non-seeded synthesis and characterization of superparamagnetic iron oxide nanoparticles incorporated into silica nanoparticles via ultrasound. Ultrasonics Sonochemistry, 23, 354-359. https://doi.org/10.1016/j.ultsonch.2014.09.011 DOI: https://doi.org/10.1016/j.ultsonch.2014.09.011
Teixeira, R. A., Costa, L. A. S., & Sant'Ana, A. C. (2019). A proposal for the adsorption of anastrozole anticancer drug on gold nanoparticle surfaces. Journal of Raman Spectroscopy, 50(10), 1462-1467. https://doi.org/10.1002/jrs.5641 DOI: https://doi.org/10.1002/jrs.5641
Toto, E., Laurenzi, S., & Santonicola, M. G. (2022). Recent trends in graphene/polymer nanocomposites for se-nsing devices: Synthesis and applications in environmental and human health monitoring. Polymers, 14(5), 1030. https://doi.org/10.3390/polym14051030 DOI: https://doi.org/10.3390/polym14051030
Verma, N. K., Crosbie-Staunton, K., Satti, A., Gallagher, S., Ryan, K. B., Doody, T., McAtamney, C., MacLoughlin, R., Galvin, P., Burke, C. S., & Volkov, Y. (2013). Magnetic core-shell nanoparticles for drug delivery by nebulization. Journal of Nanobiotechnology, 11(1), 1-12. https://doi.org/10.1186/1477-3155-11-1 DOI: https://doi.org/10.1186/1477-3155-11-1
Yang, K., Peng, H., Wen, Y., & Li, N. (2010). Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Applied Surface Science, 256(10), 3093-3097. https://doi.org/10.1016/j.apsusc.2009.11.079 DOI: https://doi.org/10.1016/j.apsusc.2009.11.079
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Muhammad Ahsan, Sobia Qasim, Ajmal Shah, Nelofar, Irum Nawaz, Muhammad Kashif, Wisal Ahmad

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
1) Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
2) Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
3) Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.


